Assessment of Social Transmission of Food Preferences Behaviors
Summary
This paper presents a protocol for the investigation of social transmission of food preference in mice. The advantages and possible applications for this procedure, for instance, in detecting early changes in AD mouse models, are highlighted. To conclude, interpretation of the results in light of critical details are discussed.
Abstract
Olfactory recognition deficits are suggested to be able to serve as clinical marker to differentiate Alzheimer's disease (AD) subjects from healthy aging groups. For example, olfactory dysfunction in AD can present as impairment in olfactory recognition, emerging during early stages of the disease and worsening while the disease progresses. The social transmission of food preferences (STFP) task is based on a rudimentary form of communication between rodents concerning distant foods dependent on the transmission of olfactory cues. Healthy wild-type mice would prefer to eat a novel, flavored food that was previously cued by a conspecific, and this food preference would be hampered in transgenic AD mice, such as the APP/PS1 model. Indeed, a strong preference for the cued food in C57Bl6/J mice of 3 months of age was found, and this was reduced in 3 months old transgenic APP/PS1 mice. In summary, STFP task could be a powerful measure to be integrated in present subclinical detection assays of AD.
Introduction
Olfactory recognition deficits are suggested to serve as clinical marker to differentiate Alzheimer's disease (AD) subjects from normal aging groups1,2,3,4. A range of neuropsychiatric disorders are characterized by disturbances in olfactory recognition and memory, including AD and Parkinson's5,6. Several behavioral tests and protocols have been established to assess olfactory recognition and discrimination in animal models7. As such, translational research using appropriate and validated animal models and tests for olfactory memory may advance better diagnosis and treatment for neurodegenerative disorders. Therefore, the social transmission of food preference (STFP) test that was originally invented in the early 80's8 was adapted. In this task, animals are evaluated on their innate ability to learn about food safety from their conspecifics. Underlying select-reject decision, processes involve assessment of the sensory characteristics of the food and an animal must be able to review and ingrate different features (i.e., taste and odor).
The STFP test consists of a rather simple phenomenon: after interaction of the naive 'observer' rodent with a 'demonstrator' that previously consumed a food, the observer normally demonstrates a greater preference for this food8,9,10. Analyzing necessary conditions resulting in this preference, showed that direct subject – demonstrator exposure (eaten or dusted with a food) is enough to boost the observer's preference. However, purely smelling nor eating a food are not sufficient to induce this type of preference11,12.
The STFP protocol consists of four steps over five days. The first step consists of increasing the motivation of the animals to make them eat a novel food. To do so, all rodents are put on a 23-h, food-deprivation schedule, receiving regular chow for 1 h/day for two consecutive days. In the second step, the priming phase, each demonstrator is provided for 1 h with food containing a novel flavor (chow mixed with either cocoa or cinnamon in the original experiments). In the third step, the social interaction phase, each demonstrator is placed into the cage of a subject observer rodent for 30 min. In the fourth step, 24 h after the social interaction, each subject is offered the choice of both flavored diets. The observer's intake of both foods and preference percentages of both diets eaten by the subject are assessed.
Selective neurotoxic lesions of hippocampus-subiculum are shown to impair performance on this task13. Also, mutations affecting hippocampal function in mice have been reported to prevent food preferences14,15,16,17. Importantly, STFP performance does not exclusively rely on proper hippocampal functioning. It was reported in several pharmacological, genetic manipulations, and lesion studies that other brain structures beside hippocampus may play a role in mediating different aspects of socially-induced diet choice learning and memory. For instance, cholinergic neurons of the medial septum/vertical limb of the diagonal band or nucleus basalis magnocellularis/substantia innominata are suggested to possess different roles in acquiring and retrieving non-spatial social memory of olfactory cues18. Furthermore, orbitofrontal cortex has been implicated in odor-guided learning, and cholinergic depletion of the entire neocortex resulted in STFP deficits, indicating that these brain regions are essential for this type of associative learning19.
Possible confounding factors were avoided as much as possible, such as spreading of the odors or dragging of the food outside the cups. An additional habituation step to the apparatus and a supplementary test before the actual STFP task were added, to assess if the rodents can actually smell and are willing to eat novel foods, the buried cookie test20. Also, by including automated video-tracking, the time spent exploring both the demonstrator during the social interaction as well as the food during the test phase could also be measured. The exploration of the path for each subject is recorded using a camera connected to a video tracking-software equipped computer. As such, different aspects of exploration performance, such as time in each zone, and number of zone visits can be calculated. This gives more detailed information about the animals' activity during the test phase, besides the amount of consumed food as in the original STFP protocol.
In previous experiments with an AD mouse model, the THY-Tau22 model, it was found that impairment in this STFP memory from the age of 9-10 months could be picked up, and these co-occurred with deficits in hippocampal synaptic plasticity and tau pathology in the hippocampus16. Since tau pathology occurs late in disease progression, according to the amyloid cascade hypothesis after the disposition of amyloidal plaques21, it was hypothesized that STFP deficits could be detected at an earlier age in amyloid transgenic mice. Therefore, the STFP test was applied in 3 months old APP/PS1 mice22, the most common used model of AD. This type of socially-induced food choice was indeed found to be impaired in the APP/PS1 mice. It is important that these mice, at least at this age, were devoid of general olfactory, locomotor, or social exploration impairments. To conclude, olfactory recognition dysfunction could be an important early screening method for AD in humans and mice alike. This reliable, cheap and easy screening for early AD could be useful for therapeutic research. If we could screen for early AD more effectively, we could interfere earlier on in the disease process.
Protocol
All protocols have been reviewed and approved by the animal experiments committee of the University of Leuven, Belgium, and were carried out in accordance with the European Community Council Directive (86/609/EEC).
1. Equipment, Apparatus, and Room Set Up:
- Prepare a Crawley's three-chamber box23 which consists of three chambers (19 x 45 cm, height 30 cm) with transparent dividing walls allowing free access to each chamber.
- Utilize two food cups made from transparent plastic (diameter of 3.8 cm, height of 3.2 cm) and place them inside fitting food cup holders consisting of a metal ramp (10×10 cm, height 7 cm), with a center hole fitting the food cups.
- Use a wire cup-like container large enough to hold a mouse (diameter of 12 cm, height 10 cm). Place it in the middle chamber for the demonstrator in phase 6.
- Clean the apparatus after each trial with 70% ethanol to prevent olfactory traces between mice and let it dry out.
NOTE: Behavioral testing is preferably done between 9:00 am and 6:00 pm. General room lighting in the apparatus and experimental room is 650 lux. Use a video-tracking software program to record mouse activity.
2. Flavored Food Preparation:
- Take regular food chow pellets and add 2% of celery or paprika. Mix the food well and put it in food containers for further use.
3. Motivational Phase (day 1-2):
- Put all rodents on a 23-h, food-deprivation schedule, receiving their regular chow for 1 h/day for two consecutive days in a room with a 12 h light/dark cycle with ad libitum access to water. Turn the light on 8 am.
NOTE: In the original protocol, during the priming phase, each demonstrator was provided, for 1 h, with food containing a novel flavor. We gave the demonstrators the flavored-food already during their 1 h feeding time, so in total 2 h instead of the original 1 h is used. - For the demonstrator mouse, use a mouse of the same background, age, gender and weight, without any prior contact (not littermates) with the subject / observer mouse.
NOTE: The same demonstrator mice may be used between social transmission trials.
4. Buried Cookie Test (day 3):
- Before starting the STFP procedure, evaluate the olfactory function of the subjects using the buried cookie test20. Bury a chocolate chip cookie approximately 1 cm beneath the surface in a random corner of a clean individual housing cage.
- Next, place the observer mouse into the cage and record the latency to find the cookie with a 15 min cut-off. Do not let the mouse eat the cookie.
NOTE: This simple test can rule out olfaction detection deficits and prevent time-consuming unnecessary STFP testing.
5. Habituation Phase (day 3):
- Separate the right and left compartments by lowering the walls.
- Put two food containers, filled with food pellets (regular food chow) in each corner of the three-chamber-apparatus.
- Gently place the observer mouse in the center chamber.
- Let the subject mouse habituate to the apparatus for 10 min.
- After 10 min, open the sliding doors and let the subject mouse freely explore the whole apparatus for 10 min.
NOTE: Mice should remain on a food-deprived schedule during this phase.
6. Social Transmission Phase (day 4):
- Separate the right and left compartments by lowering the walls.
- Put a demonstrator mouse inside a wire containment cup, and place it in the center chamber. On the lid of the wire cup facing the mounted camera, write the abbreviation of the food which the demonstrator has been cued with.
- Gently put the subject mouse in the center chamber and let it freely explore the demonstrator for 30 min, while recording the duration (s) and number of contacts between the subject mouse and the containment cup housing the demonstrator mouse (within an area of 5 cm around the cup).
7. Food Preference Test Phase:
- Prepare two food pots for each mouse. Weigh each mouse and label it with observer ID and flavor.
- Remove the wall dividers and place the two food containers, filled with either chow containing the cued or a novel (uncued) food, in each corner of the three-chamber- apparatus (Figure 2).
- Gently place the subject mouse in the middle chamber and let it freely explore the whole arena for 120 min, while recording the duration (s) and number of contacts between the subject mouse and both the food cups (within an area of 5 cm around the cups).
- Measure the food preference by weighing the remaining food (g).
- If spillage occurs, put all food back into the food cup and weigh it together with the remaining food in the food cup. Spillage inside the middle chamber should be disregarded.
8. Statistical Analysis:
- Use a video-tracking program to evaluate the mouse behavior including the number of approaches, duration of approaches between subject and demonstrator mouse during phase 6 and the time that the observer mouse spent with both food cups during phase 7.
- Calculate the percentage of time spent within the proximity of the cued and uncued food, and the percentage of the eaten cued and uncued food.
- Analyze the data by one-way analysis of variance (ANOVA).
Representative Results
An example of the social exploration phase is shown in Figure 1A-B. This phase allows estimation of social exploration of the subject mouse with the demonstrator mouse that just consumed a flavored food. In the original protocol, mice were allowed to explore each other freely. In the protocol described in this paper, the demonstrator mouse was contained in a wire cup allowing nose and ano-genital contact. Also, the arena where the mice encounter each other is small (centre chamber of the three-chamber box measures 19 x 45 cm). As such, we increase the probability of the subject mouse to explore the containment cup containing the demonstrator mouse. Comparing wild-type mice to APP/PS1 mice, we found no significant differences between the total distance moved (Figure 1A), nor the time spent (Figure 1B) with the demonstrator mouse, indicating normal sociability in this AD model. The actual food preference test phase is designed to estimate socially-induced food memory (Figure 1C-D). In this phase, the subject mouse can freely choose between the cued flavored food and a novel uncued food. Typically, a WT animal remembers its previous contact with the odor, and prefers to eat more and spend more time with the matching flavored food, indicating intact socially-induced food memory (Figure 1C-D). Unlike wild-type mice, APP/PS1 mutant animals do not show preference for the cued food over the novel, uncued food (Figure 1C-D). Indifferent behavior of APP/PS1 mice in this test is indicative of decreased socially-induced food memory.
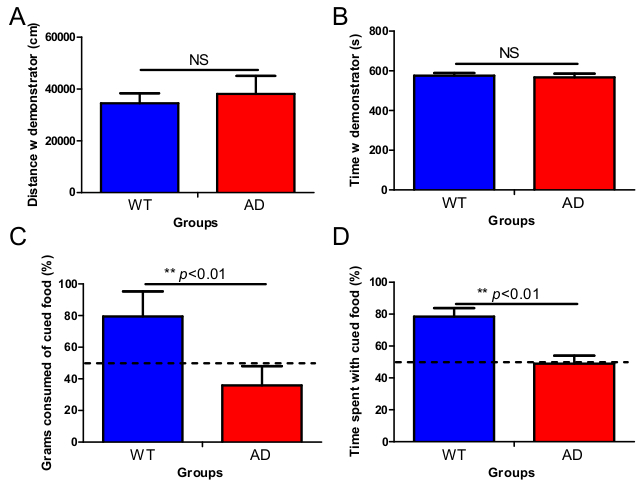
Figure 1. Social exploration of the demonstrator mouse is not different between 3 months old wild-type and APP/PS1 mice, but a loss of socially-induced olfactory memory in APP/PS1 mice was found. A. WT mice as well as AD mice traveled equal distances around the cage with the demonstrator mouse. B. Also, the time both genotypes spent around the cage holding the demonstrator was not significantly different. C. STFP test revealed that WT mice showed a clear preference for the cued food 1 day following the social encounter compared to AD mice that ate on average equal amounts of the presented food from both cups. D. This pattern was also reflected in the time the mice spent exploring the cued and non-cued foods: WT mice spent a significant higher amount of their time exploring the cued food compared to AD mice. E-F. APP/PS1 mice did not eat less than WT mice in general. (Mean ± SEM is given, WT=wild-type, AD= Alzheimer's disease, n=10 in each group, NS= non-significant, double asterisk p< 0.01).

Figure 2. Experimental Setup during the test phase. A. Picture taken from aside of the set-up with the 3-chambers and the food pots in the ramps at both ends and a mouse exploring one of them. B. Picture taken from above as captured by a video-tracking program using predefined arenas for automated and video-controlled analysis of the behaviors. Please click here to view a larger version of this figure.
Discussion
An animal behavioral repertoire can be broadly tracked back to four basic reasons, locating food and water, avoiding predators, socially interacting, and reproducing. Implementation of a behavioral pattern produced by one individual of a social group that directly promotes adoption and exhibition by another is coined social transmission24. This kind of behavior can profoundly impact a species' ecology because behavior initiated by (some) individual(s) may quickly extend out to others in the social network, like olfactory information25. Transmission of this type of information from one animal to the next can be mechanistically very simple. For example, Mus musculus is a social species comprehensively engaging in socially-induced behaviors and reciprocal social interactions. Here, we present a test to evaluate socially-induced olfactory memory in mice. The STFP test was originally developed by Galef and Wigmore, and has been gaining more and more interest over the past years. Later studies using similar procedures included refinements to improve data reliability for analysis of sociability and olfactory memory and preferences26,27. The main specificity of this test is the social transmission phase, in which a subject/observer mouse encounters a demonstrator animal that has been fed a food containing a novel flavor. During this single, social encounter, demonstrators and observers are able to exchange information about the food. 24 h after the interaction, the observer mice have to choose between the cued food or a novel food. As such, STFP can be used to study long-term memory in animals.
Containing the demonstrator mouse has several advantages over free interaction in previous versions of STFP protocols. For instance, aggression, which sometimes occurs in transgenic AD mouse models, is limited. Also, it prevents sexual interactions. Having the demonstrator contained could thus be a less aversive option for the observer mouse to interact, which in the case of an AD mouse is sometimes found to be less sociable and reluctant to interact with freely moving mice. The disadvantage is that the wire cup itself needs to be cleaned each time in between mice and can be regarded as an aversive stimulus itself.
This test does not require an animal to perform complex or non-natural behavior like many operant conditioning tasks, or the Morris water maze. Although this test is relatively time-consuming, it allows monitoring and recoding of multiple repertoires of specific parameters, like sociability during the social transmission phase. Or by simply repeating the STFP test phase, mice can be retested with a longer delay. A few considerations should be kept in mind regarding the animal mode. Observed (social) anxiety can be caused by insufficient habituation of the observer mice to the apparatus or set-up, or because of genetic modification or disease induced neophobia. For example, alterations in sociability are a core characteristic of depression, autism spectrum disorder and schizophrenia28,29,30. Thus, the test should be carefully used in animal models of these diseases. Animal models that are shown to be sensitive to dietary alterations or food deprivation should be avoided. To conclude, impaired odor recognition behavior has been implicated as a distinctive feature for several psychiatric disorders. Therefore, the full potential of the STFP test remains to be discovered.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Research supported by FWO postdoctoral fellowship to AVdJ. The authors would like to thank Leen Van Aerschot and Ilse Bloemen for their technical support.
Materials
| 3-chamber apparatus | custom made | 19 x 45 cm, height 30 cm | |
| wire cages to hold mouse | custom made | diameter of 12 cm, height 10 cm | |
| ramp to hold food cup | custom made | 10 x 10 cm, height 7 cm | |
| food cups | sunlessbody via ebay | http://www.ebay.com.au/itm/Plastic-Sample-Jars-Pots-Cups-Containers-with-Hinged-Lid-x-200-Small-25ml-/251708415240 | diameter of 3.8 cm, height 3.2 cm |
| lux meter | Volcraft | BL-10 L 0 – 40000 lx | |
| paprika herb | Delhaize | ID:716703 | Gemalen paprika,| 40 g |
| celery herb | Delhaize | ID:716301 | Selderzout, 57 g |
| vide-tracking software | Ethovision (Noldus) | http://www.noldus.com/animal-behavior-research/products/ethovision-xt | |
| ANYmaze (Stoelting) | https://www.stoeltingco.com/any-maze-video-tracking-software-1218.html |
Riferimenti
- Nordin, S., Murphy, C. Odor memory in normal aging and Alzheimer’s disease. Ann Ny Acad Sci. 855, 686-693 (1998).
- Devanand, D. P., et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiat. 157 (9), 1399-1405 (2000).
- Peters, J. M., Hummel, T., Kratzsch, T., Lötsch, J., Skarke, C., Frölich, L. Olfactory function in mild cognitive impairment and Alzheimer’s disease: an investigation using psychophysical and electrophysiological techniques. Am J Psychiat. 160 (11), 1995-2002 (2003).
- Bahar-Fuchs, A., Moss, S., Rowe, C., Savage, G. Awareness of olfactory deficits in healthy aging, amnestic mild cognitive impairment and Alzheimer’s disease. Int Psychogeriatr. 23 (7), 1097-1106 (2011).
- Serby, M., Larson, P., Kalkstein, D. The nature and course of olfactory deficits in Alzheimer’s disease. Am J Psychiat. 148 (3), 357-360 (1991).
- Hidalgo, J., Chopard, G., Galmiche, J., Jacquot, L., Brand, G. Just noticeable difference in olfaction: discriminative tool between healthy elderly andpatients with cognitive disorders associated with dementia. Rhinology. 49 (5), 513-518 (2011).
- Sánchez-Andrade, G., James, B. M., Kendrick, K. M. Neural encoding of olfactory recognition memory. J Reprod Develop. 51 (5), 547-558 (2005).
- Galef, B. G., Wigmore, S. W. Transfer of information concerning distant foods: A laboratory investigation of the ‘information-centre’ hypothesis. Anim Behav. 31, 748-758 (1983).
- Galef, B. G. Social interaction modifies learned aversions, sodium appetite, and both palatability and handling-time induced dietary preference in rats (Rattus norvegicus). J Comp Psychol. 100 (4), 432-439 (1986).
- Galef, B. G., Kennett, D. J., Stein, M. Demonstrator influence on observer diet preference: Effects of simple exposure and the presence of a demonstrator. Anim Learn Behav. 13, 25-30 (1985).
- Galef, B. G., Kennett, D. J. Different mechanisms for social transmission of diet preference in rat pups of different ages. Dev Psychobiol. 20 (2), 209-215 (1987).
- Galef, B. G. Enduring social enhancement of rats’ preferences for the palatable and the piquant. Appetite. 13, 81-92 (1989).
- Bunsey, M., Eichenbaum, H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 5 (6), 546-556 (1995).
- Mayeux-Portas, V., File, S. E., Stewart, C. L., Morris, R. J. Mice lacking the cell adhesion molecule Thy-1 fail to use socially transmitted cues to direct their choice of food. Curr Biol. 10 (2), 68-75 (2000).
- McFarlane, H. G., Kusek, G. K., Yang, M., Phoenix, J. L., Bolivar, V. J., Crawley, J. N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 7 (2), 152-163 (2008).
- Van der Jeugd, A., et al. Hippocampal tauopathy in tau transgenic mice coincides with impaired hippocampus-dependent learning and memory, and attenuated late-phase long-term depression of synaptic transmission. Neurobiol Learn Mem. 95 (3), 296-304 (2011).
- Koss, D. J., et al. Mutant Tau knock-in mice display frontotemporal dementia relevant behaviour and histopathology. Neurobiol Dis. 91, 105-123 (2016).
- Vale-Martínez, A., Baxter, M. G., Eichenbaum, H. Selective lesions of basal forebrain cholinergic neurons produce anterograde and retrograde deficits in a social transmission of food preference task in rats. Eur J Neurosci. 16 (6), 983-998 (2002).
- Ross, R. S., McGaughy, J., Eichenbaum, H. Acetylcholine in the orbitofrontal cortex is necessary for the acquisition of a socially transmittedfood preference. Learn Memory. 12 (3), 302-306 (2005).
- Mu, Y., Crawley, J. N. Simple Behavioral Assessment of Mouse Olfaction. Curr Protoc Neurosci. 8, 24-34 (2009).
- Hardy, J. A., Higgins, G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 256 (5054), 184-185 (1992).
- Radde, R., et al. ABeta42 driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 7 (9), 940-946 (2006).
- Nadler, J. J., et al. Automated apparatus for quantification of social behaviors in mice. Genes Brain Behav. 3 (5), 303-314 (2004).
- Galef, B. G. Imitation in animals: history, definition, and interpretation of data from the psychological laboratory. Social learning: psychological and biological perspectives. , (1988).
- Pulliam, H. R., Melgren, R. On the theory of gene-culture co-evolution in a variable environment. Animal cognition and behavior. , 427-443 (1983).
- Wrenn, C. C., Harris, A. P., Saavedra, M. C., Crawley, J. N. Social transmission of food preference in mice: Methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 117 (1), 21-31 (2003).
- Singh, A., Kumar, S., Singh, V. P., Das, A., Balaji, J. Flavor Dependent Retention of Remote Food Preference Memory. Front Behav Neurosci. 2 (11), 7-17 (2017).
- Kazdoba, T. M., Leach, P. T., Crawley, J. N. Behavioral phenotypes of genetic mouse models of autism. Genes Brain Behav. 15 (1), 7-26 (2006).
- Riedel, G., Kang, S. H., Choi, D. Y., Platt, B. Scopolamine-induced deficits in social memory in mice: reversal by donepezil. Behav Brain Res. 204 (1), 217-225 (2009).
- Naert, A., et al. Behavioural alterations relevant to developmental brain disorders in mice with neonatallyinduced ventral hippocampal lesions. Brain Res Bull. 94, 71-81 (2013).

