Generation of Immature, Mature and Tolerogenic Dendritic Cells with Differing Metabolic Phenotypes
Summary
Immature dendritic cells can be selectively differentiated into tolerogenic or mature dendritic cells to regulate the balance between immunity and tolerance. This work presents a means to generate from immature monocyte derived dendritic cells (moDCs), in vitro tolerogenic and mature moDCs that differ in metabolic phenotypes.
Abstract
Immune response results from a complex interplay between the antigen non-specific innate immune system and the antigen specific adaptive immune system. The immune system is a constant balance in maintaining tolerance to self-molecules and reacting rapidly to pathogens. Dendritic cells (DCs) are powerful professional antigen presenting cells that link the innate immune system to the adaptive immune system and balance the adaptive response between self and non-self. Depending on the maturation signals, immature dendritic cells can be selectively stimulated to differentiate into immunogenic or tolerogenic DCs. Immunogenic dendritic cells provide proliferation signals to antigen-specific T cells for clonal expansion; while tolerogenic dendritic cells regulate tolerance by antigen-specific T-cell deletion or clonal expansion of regulatory T-cells. Due to this unique property, dendritic cells are highly sought after as therapeutic agents for cancer and autoimmune diseases. Dendritic cells can be loaded with specific antigens in vitro and injected into the human body to mount a specific immune response both immunogenic and tolerogenic. This work presents a means to generate in vitro from monocytes, immature monocyte derived dendritic cells (moDCs), tolerogenic and mature moDCs that differ in surface marker expression, function and metabolic phenotypes.
Introduction
DC was first described by Paul Langerhans (Langerhans cells) in the late nineteenth century as referenced by Jolles 1 and characterized by Ralph Steinman and Zanvil Cohn in 1973 who recognized them as professional antigen presenting cells 2. DCs are found in peripheral blood and in most tissues of the body, especially abundant in tissues that are exposed to the external environment such as the skin (present as Langerhans cells) and in the linings of the nose, lungs, stomach and intestines which enable them to encounter extrinsic antigens. Immature DCs have endocytic capability but relatively low capacity to stimulate T cells 3. Immature DCs express various pattern recognition receptors (PRRs) that capture pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) 4. Activating danger signals drive maturation towards immunogenic DCs while self-molecules result in T cell unresponsiveness and apoptosis 5. Immunogenic DCs are characterized by the upregulation of MHC molecules and co-stimulatory surface molecules and their ability to prime naive T cells 6,7.
Immature DCs can also be matured towards a Treg-inducing or tolerogenic state in response to Vitamin D3 metabolite 1a,25(O)2D3 and certain immunosuppressive agents like Interleukin-10 (IL-10), dexamethasone and rapamycin 8-9. Tolerogenic DCs are characterized by their expression of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) containing surface receptors and ligands. Signal transduction of ITIMs containing ILT family members, ILT3 and ILT4 in tolerogenic DCs inhibit alloproliferation and drive Foxp3+ Treg expansion 10,11. These unique properties of tolerogenic DCs lead to their profound potency in vivo, namely the ability to induce durable tolerance to transplanted allogeneic grafts and suppress the development of autoimmune diseases. Tolerogenic DCs may be viewed therefore as a subtype of mature-polarized DCs which function in the inhibition of immune activation.
Currently, there are two general subsets of dendritic cells in human peripheral blood: plasmacytoid DCs and myeloid DCs 12. Circulating DCs are rare constituting to less than 2% of leukocytes in human blood and this poses a difficulty to the isolation of an appropriate number of DCs to study their immunoregulatory functions. To overcome this problem, monocyte differentiated DCs are used as an in vitro model for the study of dendritic cell function. These in vitro DCs have similar receptors and functions compared to in vivo DCs. Detailed comparison of in vivo DCs and in vitro generated monocyte derived DCs (moDCs) are investigated by other laboratories 13,14,15. It is also reported that moDCs and CD1c+ DCs were equivalent at antigen presenting and inducing T cell function15.
In this paper, we describe a method of generating immature moDCs from peripheral blood monocytes and then differentiating them into immunogenic and tolerogenic DCs. These monocyte derived dendritic cells (moDCs) are characterized by their surface markers, cytokine profile, immunoregulatory functions and metabolic states. Immunogenic and tolerogenic dendritic cells produce different cytokines which result in expansion of either allogenic T cells or regulatory T cells. In this paper, cytokine profiling is performed with systems using multiplex technology. Growth medium of cells are incubated with antibody immobilized color coded beads and read in a compact analyzer. Metabolic states of DCs are analyzed using extracellular flux analyzers that measure oxygen consumption rate, an indicator of cellular respiration, and extracellular acidification rate which reflects glycolytic flux in dendritic cells. Measurement of these bioenergetics rates provides a means to track the changes in cellular metabolism which are vital in dendritic cell development and function.
Protocol
This research was approved by the Institutional Review Board (NUS-IRB 10-250).
1. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
- Preparation of Reagent
- Prepare PBS/EDTA: phosphate-buffered saline solution (PBS) and supplement with 2 mM ethylenediaminetetraacetic acid (EDTA). Sterilize this solution by filtration through a 0.2 µm filter. Note to store PBS/EDTA at 4 °C and warm to room temperature before use.
- Prepare staining buffer: phosphate-buffered saline solution (PBS) supplement with 2% fetal bovine serum (FBS), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 2 mM ethylenediaminetetraacetic acid (EDTA). Sterilize this solution by filtration through a 0.2 µm filter.
- Collect Blood from Blood Cone
Note: The blood cone contains white blood cell components collected after plateletpheresis from hospital. If blood is collected in heparin or EDTA tubes, dilute blood with PBS in 1:1 ratio and proceed to step 1.3; if buffy coat is received, dilute buffy coat with PBS in 1:2 ratio and proceed to step 1.3.- Cut the two ends of the cone to allow blood to flow out into a 50 ml tube. Note that the cone usually contains 10 ml of blood.
- Use a blunt end syringe containing 30 ml of PBS/EDTA to wash the cone and collect in a 50 ml tube.
- Dilute blood further with PBS/EDTA to a final volume of 80 ml.
- Isolation of PBMCs by Density Centrifugation16
- Aliquot 15 ml Ficoll each to 4 fresh 50 ml tubes.
- Use a 25 ml serological pipet to add 20 ml of diluted blood over the Ficoll layer. Take note to hold the 50 ml tube at a 45 angle and take care to not disturb the interphase.
- Centrifuge the tubes at 805 x g without brakes for 30 min, 20 °C.
- Remove the plasma layer and collect the ring of PBMCs lying just below the plasma layer with a Pasteur pipet. Combine four tubes of PBMCs into two 50 ml tubes. Note to avoid collecting the transparent layer below the PBMCs.
- Add PBS/EDTA to a final volume of 50 ml per tube of PBMCs and centrifuge at 548 x g with brakes for 10 min, 20 °C.
- Aspirate supernatant and resuspend pellet in each tube with 25 ml of staining buffer and combine into one 50 ml tube.
- Centrifuge at 367 x g with brakes for 5 min, 4 °C.
- Aspirate supernatant and resuspend pellet with 10 ml of staining buffer.
2. Monocyte Enrichment by Magnetic Separation17
- Determine Cell Number
- Take 20 µl of PBMC cell suspension and mix with 20 µl of trypan blue to count the number of live cells using cytometer.
- Centrifuge cell suspension at 367 x g with brakes for 10 min, 4 °C.
- Aspirate supernatant completely and resuspend cell pellet to a concentration of 107 cells per 80 µl of staining buffer.
- Magnetic Labeling
- Add 20 µl of CD14 microbeads per 107 cells, mix well and incubate for 15 min at 2 – 8 °C.
- Wash cells by adding 1 ml of staining buffer per 107 cells and centrifuge at 367 x g with brakes for 10 min, 4 °C.
- Aspirate supernatant completely and resuspend cell pellet in a concentration of 107 cells per 50 µl of staining buffer.
- Magnetic Separation
- Use medium size column for a maximum of 2 x 108 total cells.
Note: Choose an appropriate column and separator according to the number of total cells and the number of CD14+ cells recommended in the datasheet. - Place column in the magnetic field of a suitable separator and prepare column by rinsing with 500 µl of staining buffer.
- Pipette cell suspension onto column and collect unlabeled cells that pass through the column with a 15 ml tube.
- Replace a new 15 ml tube under column and wash column 3 times with 500 µl of staining buffer. Make sure the column reservoir is empty before adding new staining buffer between washes.
- Remove column from the separator and place it on a fresh 15 ml tube.
- Pipette 1 ml of staining buffer onto the column. Immediately flush out the magnetically labelled cells by firmly pushing the plunger (provided in the kit) into the column.
- Repeat steps 2.3.2 to 2.3.6 using the eluted fraction on a new column to increase the purity of CD14+ cells. Note that the beads will be released from the cells automatically in culture during step 3.2.
- Use medium size column for a maximum of 2 x 108 total cells.
3. Differentiation of Dendritic Cells to Different Activation States
- Preparation of Reagent (Endotoxin Level has to be Less than 0.1 EU/ml in all Reagents)
- Prepare cell culture medium: RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 1% Non-Essential Amino Acids (NEAA) and 0.05 mM 2-mercaptoethanol (2ME). Sterilize this solution by filtration through a 0.2 µm filter.
- Prepare cytokines: reconstitute IL-4 and GM-CSF in cell-culture grade water to a concentration of 0.25 mg/ml respectively under aseptic conditions. Aliquot cytokines into 200 µl microcentrifuge tubes and store at -80 °C.
- Prepare vitamin D3 stock: reconstitute vitamin D3 in cell-culture grade water to a concentration of 100 mM under aseptic conditions. Aliquot vitamin D3 into 1.5 ml microcentrifuge tubes and store at -20 °C.
- Prepare dexamethasone stock: reconstitute dexamethasone in cell-culture grade water to a concentration of 10 mM under aseptic conditions. Aliquot dexamethasone into 1.5 ml microcentrifuge tubes and store at -20 °C.
- Prepare LPS: reconstitute Lipopolysaccharides (LPS) in cell-culture grade water to a concentration of 1 mg/ml under aseptic conditions. Aliquot LPS into 1.5 ml microcentrifuge tubes and store at -20 °C.
- Generating Different moDCs
- Seed 4 sets of CD14+ monocytes in concentration of 0.3 – 0.5 x 106/ml of cell culture medium supplemented with 200 ng/ml of GM-CSF and 200 ng/ml of IL-4 in 6 well plates. Note that this is Day 0 and the volume of medium in each 6 well is 2 ml.
- Incubate cells in tissue culture incubator at 37 °C with 5% CO2.
- Remove 850 µl of medium from the culture at Day 4 and centrifuge at 300 x g for 5 min, 4 °C. Aspirate supernatant and resuspend pellet in 1 ml of cell culture medium containing 2x of (200 ng/ml of GM-CSF and 200 ng/ml of IL-4). Add this cell mixture back to the culture. Note that 2x concentration added for GM-CSF and IL-4 will become 1x when the 1 ml of medium is added back into the culture.
- Add 1 µl of vitamin D3 stock and 1µl of dexamethasone stock per ml of medium to two of the sets on Day 5 to generate tolerogenic moDCs. Note that the final concentration of vitamin D3 is 100 nM and dexamethasone is 10 nM; addition of GM-CSF and IL-4 is not required on Day 5.
- Add 200 ng/ml of GM-CSF and 200 ng/ml of IL-4 to all the sets on Day 6.
- Add 1 µg/ml of LPS to one of the sets treated with only GM-CSF and IL-4 at Day 6 to generate mature moDCs.
- Add 1 µg/ml of LPS to one set of the tolerogenic moDCs at Day 6 to generate LPS-tolerogenic moDCs.
- Harvest the different types of moDCs at Day 7 by flushing culture dish with PBS, EDTA for flow cytometry or other studies. Note that only non-adherent cells are harvested. Take note that percentage yield from CD14+ monocytes for immature moDCs, mature moDCs, tolerogenic moDCs and LPS-tolerogenic DCs are about 90%, 50%, 60% and 60% respectively and varies between blood donors and FBS lot.
4. Flow Cytometry
- Cell Surface Marker Characterization on moDCs
- Detach cells from culture dish by pipetting. Wash the cells once with PBS/EDTA and resuspend cells in a concentration of 5 x 105 cells per 50 µl of staining buffer in 1.5 ml microcentrifuge tube.
- Incubate 0.5 x 106 cell aliquots with PerCP-conjugated HLADR (1:100), PE-conjugated CD80 (1:50), PE-conjugated CD83 (1:25), PE-conjugated CD86 (1:50), APC-conjugated CD11c (1:50), PE-conjugated CD14 (1:50) and PE-conjugated BDCA3 (1:50) and APC-conjugated ILT3 (1:25) in the dark for 30 min at 4 °C. Isotype-matched PerCP-conjugated MAb (1:20), PE-conjugated MAb (1:11), APC-conjugated MAb (1:11) will serve as negative controls.
- Detect the expression levels of the surface markers using a flow cytometer 18.
- Analysis of Mitochondria Membrane Potential of moDCs
- Prepare stock solution of 1 mM Red Chloromethyl-X-rosamine (CMXRos) by adding 94.1 µl of dimethyl sulfoxide (DMSO) per 50 µg of lyophilized Red CMXRos.
- Incubate 2 x 105 moDCs with 100 nM Red CMXRos in 1 ml of Hank's Balanced Salt Solution (HBSS) for 30 min at 37 °C in 15 ml tube.
- Add 2 ml of PBS/EDTA to cells and centrifuge at 300 x g for 5 min, room temperature. Repeat 2 times. Aspirate supernatant and resuspend cell pellet in 300 µl of PBS, EDTA, 2% FCS for flow cytometry analysis. Take note to use PE channel to analyze Red CMXRos signal.
- Marker Characterization of T-cells
- Incubate 50 µl of 1.2 x 106 CFSE-labeled allo CD4+ T-cells (generated from step 5.6) with PerCP-conjugated CD3 (1:200), PE/Cy7-conjugated CD4 (1:400) and APC/Cy7-conjugated CD25 (1:100) in the dark for 30 min at 4 °C. Stain T-cells using a commercial kit with Foxp3-Alexa Fluor 647 (1:50) in the dark for 1 hr at room temperature.
5. Alloreaction Studies
- Prepare 5 mM stock solution of carboxyfluorescein succinimidyl ester (CFSE) by adding 18 µl of DMSO to lyophilized CFSE, according to manufacturer's protocol.
- Purify CD4+ T-cells from PBMCs
- Determine cell number by taking 20 µl of PBMC cell suspension and mix with 20 µl of trypan blue to count the number of live cells using a cytometer.
- Centrifuge cell suspension at 367 x g with brakes for 10 min, 4 °C.
- Aspirate supernatant completely and resuspend cell pellet in a concentration of 5 x 107 cells per 1 ml of staining buffer in a 5 ml polystyrene tube.
- Add Human CD4+ T Cell Enrichment Cocktail at 50 µl/ml cells. Mix well and incubate at room temperature (15 – 25 °C) for 10 min.
- Vortex magnetic particles for 30 sec and add magnetic particles at 100 µl/ml cells. Mix well and incubate at room temperature for 5 min.
- Add staining buffer to the cell suspension to a total volume of 2.5 ml. Mix the cells in the tube by gently pipetting up and down 2 – 3 times. Place the tube into the magnet and incubate for 5 min.
- Invert magnet with tube and decant the suspension (contains T cells) into a new 5 ml polystyrene tube.
- Determine cell number and incubate 1.2 x 106 CD4+ T-cells with 5 µM CFSE in 1 ml of PBS for 20 min at 37 °C, protected from light.
- Add five times the original staining volume of cell culture medium (prepared according to step 3.1.1) to the cells and incubate for 5 min.
- Centrifuge at 300 x g for 5 min, 4 °C and resuspend pellet in cell culture medium at a concentration of 2 x 105 per 75 µl.
- Add 75 µl of 2 x 105 CFSE-labeled CD4+ T-cells to each moDC culture containing 75 µl of cell culture medium with 0, 2.5 x 103, 5 x 103, 10 x 103, 20 x 103 and 40 x 103 moDCs in 96 well U-bottom plates. Culture for 6 days in tissue culture incubator at 37 °C with 5% CO2.
- Harvest by pipetting CD4+ T-cells into 15 ml tube and centrifuge at 300 x g for 5 min, 4 °C. Collect supernatant for cytokine analysis and resuspend cell pellet in 2 ml of PBS/EDTA and centrifuge at 300 x g for 5 min at 4 °C.
6. Cytokine Analysis19
- Collect supernatants from alloreaction studies as described in step 5.5.
- Preparation of Reagent for Human Cytokine/Chemokine Magnetic Panel Analysis
- For preparation of mixing bottle of antibody-immobilized beads for individual vials of beads (included in the kit), vortex for 1 min. Add 60 µl from each antibody bead vial to the mixing bottle (included in the kit) and bring to a final volume of 3 ml with Bead Diluent (provided).
- For preparation of quality controls, reconstitute Quality Control 1 and Quality Control 2 (included in the kit) with 250 µl deionized water.
- For preparation of Wash buffer, dilute 30 ml of 10x Wash buffer (included in the kit) with 270 ml of deionized water.
- For preparation of human cytokine Standard, reconstitute the human cytokine standard (included in the kit) with 250 µl deionized water to give a 10,000 pg/ml concentration.
- Add 50 µl of 10,000 pg/ml human cytokine standard to 200 µl of Assay buffer (provided in the kit) in a 1.5 ml microcentrifuge tube to make 2,000 pg/ml working standard. Transfer 50 µl of 2,000 pg/ml human cytokine standard to 200 µl of Assay buffer (provided in the kit) in a 1.5 ml microcentrifuge tube to make 400 pg/ml working standard.
- Transfer 50 µl of 400 pg/ml human cytokine standard to 200 µl of Assay buffer (provided in the kit) in a 1.5 ml microcentrifuge tube to make 80 pg/ml working standard. Transfer 50 µl of 80 pg/ml human cytokine standard to 200 µl of Assay buffer (provided in the kit) in a 1.5 ml microcentrifuge tube to make 16 pg/ml working standard.
- Transfer 50 µl of 16 pg/ml human cytokine standard to 200 µl of Assay buffer (provided in the kit) in a 1.5 ml microcentrifuge tube to make 3.2 pg/ml working standard. 0 pg/ml working standard has only 200 µl of Assay buffer in a 1.5 ml microcentrifuge tube.
- Pre wet filter plate (included in the kit) by pipetting 200 µl of Assay buffer (provided) into each well of the filter plate. Seal and place the filter plate on a plate shaker for 10 min at room temperature.
- Aspirate Assay buffer and add 25 µl of each Standard or Quality control into the appropriate wells.
- Add 25 µl of cell culture medium (prepared according to step 3.1.1) to the background, standards and control wells.
- Add 25 µl of Assay buffer to the sample wells and add 25 µl of sample into the appropriate sample wells.
- Vortex the mixing bottle containing antibody-immobilized beads and add 25 µl of the mixed beads to each well. Seal plate and incubate with agitation on a plate shaker overnight at 4 °C.
- Aspirate fluid and wash plate 2 times by adding 200 µl/well of Wash buffer and aspirating the fluid.
- Add 25 µl of detection antibodies (provided) into each well. Seal and incubate with agitation on a plate shaker for 1 hr at room temperature.
- Add 25 µl of Streptavidin- Phycoerythrin (provided) to each well containing the 25 µl of detection antibodies. Seal and incubate with agitation on a plate shaker for 30 min at room temperature.
- Aspirate fluid and wash plate as described in step 6.8.
- Add 150 µl/ well of Sheath Fluid to all wells and resuspend beads on a plate shaker for 5 min.
- Detect fluorescence intensity of beads using a 3D system 20. Analyze the median fluorescent intensity data using a five parameter log-logistic curve-fitting method for calculating cytokine/chemokines concentrations in samples21.
7. Real-time Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) Measurements
- Preparation of Reagent/Materials
- Prepare OCR medium: Assay medium supplemented with 25 mM glucose and 1 mM sodium pyruvate (pH 7.35). Sterilize this solution by filtration through a 0.2 µm filter. Take note that this medium is prepared without FBS because components in FBS are complex and vary from one lot to anther lot.
- Prepare ECAR medium: Base medium supplemented with 2 mM L-glutamine (pH 7.35). Sterilize this solution by filtration through a 0.2 µm filter. Take note that this medium is prepared without bicarbonate, glucose, pyruvate and FBS. Note that FBS has buffering capacity and will interfere with the ECAR reading.
- Prepare compounds for OCR measurements: Resuspend oligomycin with 630 µl of OCR medium to make 100 µM stock solution and dilute with OCR medium to 16 µM working concentration. Resuspend carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) with 720 µl of OCR medium to make 100 µM stock solution and dilute with OCR medium to 4.5 µM working concentration. Resuspend rotenone/antimycin A with 540 µl of OCR medium to make 50 µM stock solution and dilute with OCR medium to 10 µM working concentration.
- Prepare compounds for ECAR measurements: Resuspend Glucose with 3 ml of ECAR medium to make 100 mM stock solution and dilute to 80 mM working concentration. Resuspend oligomycin with 720 µl of ECAR medium to make 100 µM stock solution and dilute to 18 mM working concentration. Resuspend 2-deoxy-D-glucose with 1.5 ml of ECAR medium to make 1,000 mM stock solution.
- Pipet 200 µl of calibrant into each well of the cartridge and store at 37 °C with no CO2 one day before measurements.
- Real-time OCR Measurements
- Harvest moDCs and resuspend each type of moDCs in OCR medium at a concentration of 60 x 103 per 150 µl of OCR medium.
- Seed 60 x 103 cells/well in a poly-D-lysine-coated 96-well flat bottom plate and incubate in a non-CO2 incubator for 1 hr at 37 °C. Take note to use 50 µl of 50 ng/ml of poly-D-lysine to coat the plate for 1 hr and wash with sterile water. Keep plate dry for 2 hr at room temperature before use.
- Pipette 25 µl OCR medium into injection port A for all wells; 25 µl OCR medium containing 16 µM of oligomycin in injection port B for all wells; 25 µl OCR medium containing 4.5 µM of (FCCP) in injection port C for all wells and 25 µl OCR medium containing 10 mM rotenone/antimycin A in injection port D for all wells. Note that the final well concentration for oligomycin is 2 µM, FCCP is 0.5 µM and rotenone/antimycin A is 1 µM.
- Place plate into extracellular flux analyzer and run a complete OCR study with all moDCs simultaneously in four consecutive stages: basal respiration (after medium injection from port A), mitochondrial complex V inhibition (after drug injection from port B), maximal respiration induction (after drug injection from port C) and electron transport chain inhibition (after drug from port D) 22. Take note that there are 3 cycles in between injections and 6 min interval between measurements.
- Real-time ECAR Measurements
- Harvest moDCs and resuspend each type of moDCs in ECAR medium at a concentration of 60 x 103 per 150 µl of ECAR medium.
- Seed 6 x 104 cells/well in a poly-D-lysine-coated 96-well flat bottom plate and incubate in a non-CO2 incubator for 1 hr at 37 °C.
- Pipette 25 µl ECAR medium into injection port A for all wells; 25 µl ECAR medium containing 10 mM of glucose in injection port B for all wells; 25 µl ECAR medium containing 18 µM of oligomycin in injection port C for all wells and 25 µL OCR medium containing 1,000 mM 2-deoxy-D-glucose in injection port D for all wells. Note that the final well concentration for glucose is 10 µM, oligomycin is 2 µM and 2-deoxy-D-glucose is 100 mM.
- Place plate into extracellular flux analyzer and run a complete ECAR study with all moDCs simultaneously in four consecutive stages: basal respiration (after medium injection from port A), glycolysis induction (after drug injection from port B), maximal glycolysis induction (after drug injection from port C) and glycolysis inhibition (after drug from port D) 22.
- Analyze statistical differences using one-way ANOVA with a Tukey multiple comparison post-test.
Representative Results
Monocyte Purification and Dendritic Cell Differentiation
Monocytes were purified from PBMCs by density centrifugation of peripheral blood (Figure 1A), followed by CD14+ positive selection magnetic separation (Figure 1B) and cultured in complete medium in the presence of GM-CSF and IL-4 to obtain immature dendritic cells (Figure 2A). Addition of vitamin D3 and dexamethasone post GM-CSF and IL-4 resulted in the differentiation of immature moDCs into tolerogenic moDCs (Figure 2B). LPS was added to induce maturation of immature moDCs to mature moDCs (Figure 2C) and tolerogenic moDCs were stimulated with LPS to verify resistance to maturation (Figure 2D). The blood samples used in this work were obtained from healthy donors with previous informed consent.
moDC Characterization
Surface Marker Characterization by Flow Cytometry
Analysis of DC surface markers showed that mature moDCs expressed the highest levels of maturation markers HLA-DR, CD83 and CD86 compared to LPS-treated tolerogenic moDCs, tolerogenic moDCs and immature moDCs (Figure 3). These results demonstrated that tolerogenic moDCs were resistant to maturation as compared to immature moDCs following LPS stimulation. In addition, LPS-treated tolerogenic moDCs and tolerogenic moDCs displayed increased expression of CD14, BDCA3 (CD141) and Immunoglobulin-like transcript (ILT)3 compared to immature and mature moDCs. The tolerogenic moDCs that we generated here is consistent with previous reports 23.
Functional characterization of moDCs
moDCs induced for maturation become immunogenic and release cytokines that promote the proliferation of CD4+ T-cells. We assessed the immunogenicity of the different moDC subtypes by measuring the proliferation of co-cultured T cells. Tolerogenic moDCs were poorly immunogenic compared with mature moDCs, as shown by low alloproliferation of CD4+ T-cells (Figure 4A). Tolerogenic moDCs are characterized by their low IFN-Γ, low IL-12p40, and high IL-10 cytokine production in alloreaction co-cultures with CD4+ T-cells (Figure 4B). Furthermore, increasing the number of tolerogenic moDCs in co-cultures of mature moDCs induced allospecific CD4+ T-cells increased the frequency of CD25high Foxp3+ regulatory T-cells (Figure 4C).
Analysis of Mitochondrial Activity
Red CMXRos is used to reflect the mitochondrial activity to analyze the mitochondrial membrane potential levels in moDCs. Tolerogenic moDCs were observed to have higher mitochondrial activity compared with the other moDC differentiated subtypes (Figure 5A). Next, the rate of mitochondrial oxygen consumption (OCR) is assessed for the different moDC subtypes using a bioanalyzer. OCR measurements allow high resolution insights to the metabolic profile, providing information including but not limited to basal respiration, spare respiratory capacity, proton leak and non-mitochondrial respiration. The measurement of OCR provides a means to assess the ability of cells to respond to stress. The cells are metabolically perturbed by the addition of three different compounds in succession. The first injection is Oligomycin (ATP Coupler) which inhibits complex V of the electron transport chain (ETC), inhibiting ATP synthesis. This step distinguishes the percentage of oxygen consumed for ATP synthesis and the percentage of oxygen consumed to overcome proton leak across the inner mitochondrial membrane. The second injection is FCCP (ETC accelerator) that disrupts ATP synthesis by transporting hydrogen ions across the mitochondrial membrane instead of through the proton channel of Complex V. The collapse of the mitochondrial membrane potential leads to a rapid consumption of energy and oxygen, without the generation of ATP. FCCP treatment can be used to calculate the spare respiratory capacity of cells. Maintenance of spare respiratory capacity under stress conditions is critical to cell survival. This capacity is determined by several factors, including the availability of substrate and functional capacity of the enzymes involved in the ETC. The third injection is a combination of Rotenone, a Complex I inhibitor, and Antimycin A, a Complex III inhibitor. This combination shuts down mitochondrial respiration and OCR is observed to decrease as a result of impaired mitochondrial function (Figure 5C). Tolerogenic moDCs displayed higher basal OCR levels than mature moDCs (Figure 5D). In addition, tolerogenic, LPS-tolerogenic, and immature moDCs showed increased spare respiratory capacity compared with mature moDCs (Figure 5E).
Metabolic Characterization of moDCs
Because lactic acid and protons are released from cells during glycolysis, we analyzed the glycolytic activity of moDCs by performing a real-time analysis of the rate of extracellular acidification (ECAR) (Figure 6B). In the presence of glucose, the glycolytic rate of all moDCs increased compared with the basal stage, with mature moDCs exhibiting higher glycolytic rate than immature moDCs (Figure 6D). Tolerogenic and immature moDCs exhibited higher maximal glycolysis (induced by oligomycin in presence of glucose) compared with LPS-treated moDCs (Figure 6B). The glycolytic capacity of tolerogenic and immature moDCs was higher than mature moDCs (Figure 6E). In contrast to their high glycolytic rate, glycolytic reserve was the lowest in mature moDCs (Figure 6F).
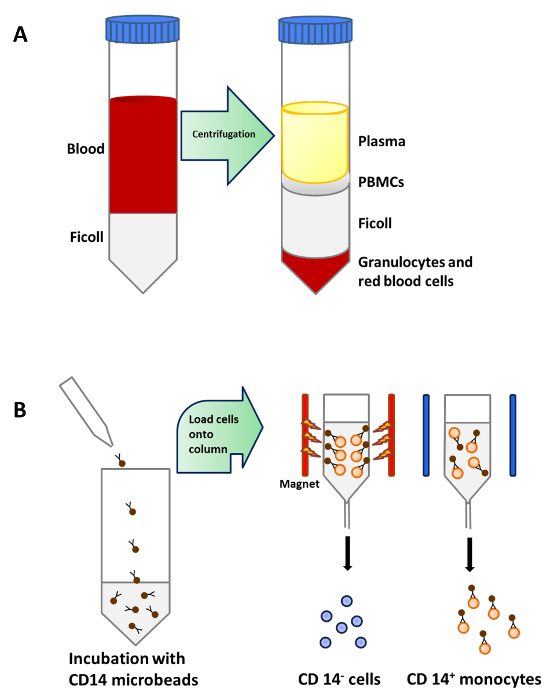
Figure 1: Monocyte Purification from Peripheral Blood. (A) 25 ml of blood is carefully layered onto 15 ml of Ficoll per 50 ml tube before centrifugation. PBMCs are concentrated in a layer below plasma after density centrifugation. (B) PBMCs are incubated with microbeads that are conjugated to monoclonal human CD14 antibodies (isotype: mouse IgG2a) and then loaded onto a column which is placed in the magnetic field of a Separator to isolate CD14+ monocytes. Please click here to view a larger version of this figure.
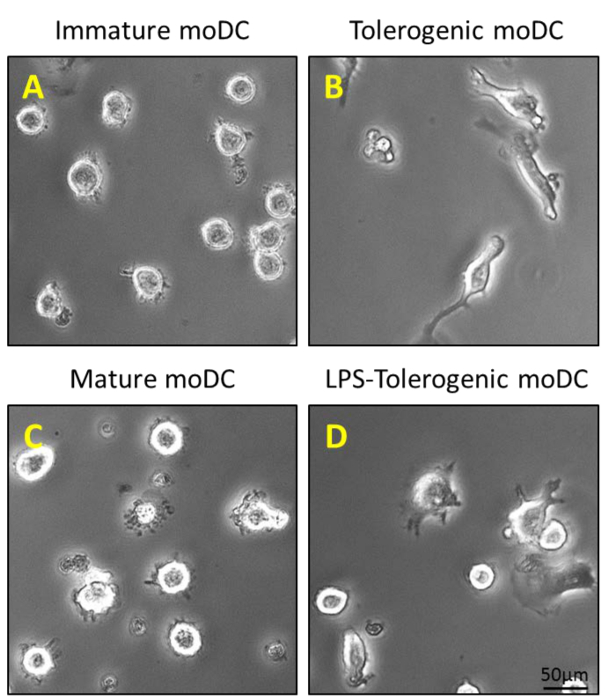
Figure 2: Morphological Characterization of moDCs. (A) 200 ng/ml of GM-CSF and IL-4 is added to purified CD14+ monocytes on Day 0, 4 and 6 to generate immature moDCs; and (B) an additional step of stimulation with 100 nM Vitamin D3 and 10 nM dexamethasone on Day 5 generates tolerogenic moDCs. Immature moDCs and tolerogenic moDCs are stimulated with 1 µg/ml LPS on Day 6 to generate (C) mature moDCs and (D) LPS-tolerogenic moDCs. Please click here to view a larger version of this figure.
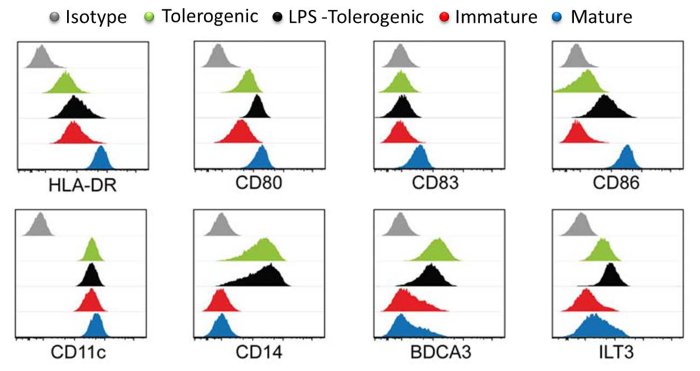
Figure 3: Surface Marker Characterization by Flow Cytometry. Expression levels of surface markers HLA-DR, CD80, CD83, CD86, CD11, CD14, BDCA3 and LT3 in tolerogenic (green), LPS-tolerogenic (black), immature (red), mature (blue) moDCs. Isotype controls are shown in grey. Individual histogram for each cell type is plotted with Y-axis cell count against X-axis log of fluorophore intensity and overlaid. All histograms are representative of four independent experiments. This figure has been modified from J Immunol 194 (11), 5174-5186, doi: 10.4049/jimmunol.1303316 (June 1, 2015). Reproduced and republished with copyright permission. Copyright 2015. The American Association of Immunologists, Inc. Please click here to view a larger version of this figure.
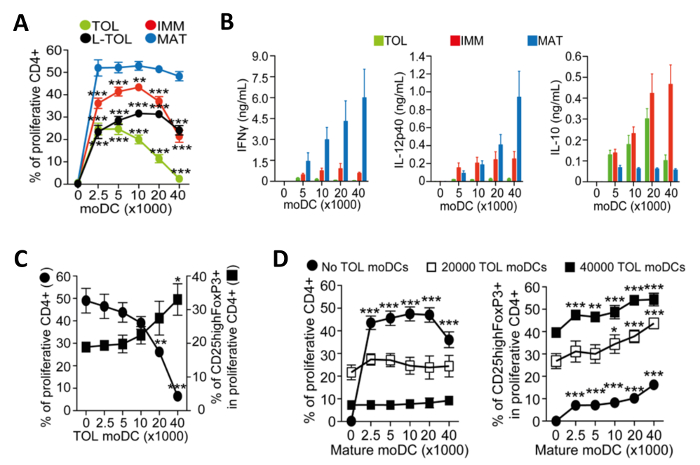
Figure 4: Functional Characterization of moDCs. (A) Quantification of alloproliferation frequency of CD4+ T-cells induced by co-culture with increasing numbers of tolerogenic (TOL; green), LPS-tolerogenic (L-TOL; black), immature (IMM; red) and mature (MAT; blue) moDCs. The data was pooled from four independent experiments; mean + S.E.M. Statistical differences between all moDCs versus mature moDCs were analyzed by two-way ANOVA with Dunnett multiple comparison post-test. (B) Cytokine analysis of IFN-Γ (left panel), IL-12p40 (middle panel) and Il-10 (right panel) in supernatants from alloreactions between CD4+ T-cells co-cultured with increasing numbers of either tolerogenic (green), immature (red) or mature (blue) moDCs. Data were pooled from six independent experiments; mean ± S.E.M. (C) CD4+ T-cell alloproliferation and regulatory T-cells expansion induced by co-culture with mature moDCs in the presence of increasing numbers of tolerogenic moDCs. Left Y-axis, frequency of CD4+ T-cell proliferation. Right Y-axis, frequency of CD25high Foxp3+ cells gated on proliferative CD4+ T-cells. The data were pooled from three independent experiments; mean ± S.E.M. Statistical differences between presence versus absence of tolerogenic moDCs were analyzed by one-way ANOVA with Dunnett multiple comparison post-test. (D) CD4+ T-cell alloproliferation (Left) and frequency of CD25highFoxP3+ cells (Right) induced by co-culture with tolerogenic moDCs in the presence of increasing numbers of mature moDCs. The data were pooled from two to three independent experiments; mean ± S.E.M. Statistical differences between the presence versus absence of mature moDCs were analyzed by two-way ANOVA with Dunnett multiple comparison post-test. This figure has been modified from J Immunol 194 (11), 5174-5186, doi: 10.4049/jimmunol.1303316 (June 1, 2015). Reproduced and republished with copyright permission. Copyright 2015. The American Association of Immunologists, Inc. Please click here to view a larger version of this figure.
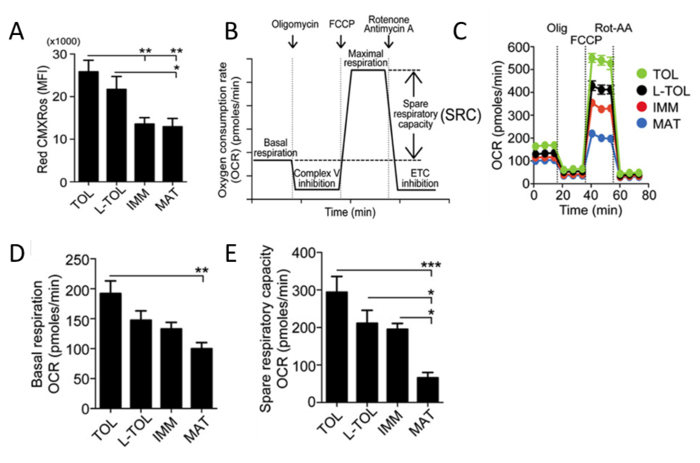
Figure 5: Analysis of Mitochondrial Activity of moDCs. (A) Levels of mitochondrial membrane potential (Red CMXRos) in moDCs were obtained by flow cytometric analysis. The data of four independent experiments were pooled. Mean ± S.E.M. (B) Schematic representation of a real-time mitochondrial respiration. OCR analysis starting from basal respiration and after the addition of oligomycin (complex V inhibition), FCCP (maximal respiration induction), and rotenone/antimycin A mixture (electron transport chain [ETC] inhibition). The mitochondrial SRC (maximal basal subtracted from maximal respiration) is derived from the OCR curve. (C) Representative kinetic study of mitochondria OCR (pmol/min) in tolerogenic (TOL, green), LPS-tolerogenic (L-TOL, black), immature (IMM, red) and mature (MAT, blue) moDCs by using sequential addition of oligomycin (Olig), FCCP, and rotenone/antimycin A (Rot-AA). (D) OCR quantification of basal respiration of moDCs and (E) spare respiratory capacity of moDCs. The data were pooled from five independent experiments. Mean ± S.E.M. This figure has been modified from J Immunol 194 (11), 5174-5186, doi: 10.4049/jimmunol.1303316 (June 1, 2015). Reproduced and republished with copyright permission. Copyright 2015. The American Association of Immunologists, Inc. Please click here to view a larger version of this figure.
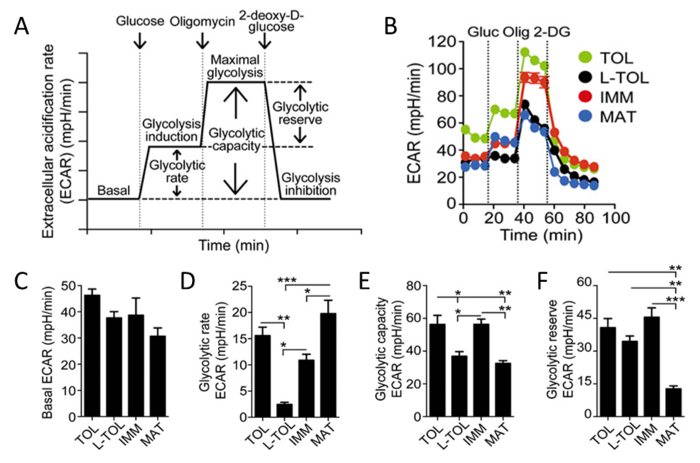
Figure 6: Metabolic Characterization of moDCs. (A) Schematic representation of real-time glycolysis. ECAR analysis starts from basal ECAR, in which the cells were incubated in glucose-free media followed by the addition of glucose (glycolysis induction), oligomycin (which induces maximal cell glycolysis and complex V inhibition), and finally 2-deoxy-D-glucose (glycolysis inhibition). Glycolytic rate (glycolysis induction subtracted for basal ECAR), glycolytic capacity (maximal glycolysis subtracted for basal ECAR), and glycolytic reserve (maximal glycolysis subtracted for glycolysis induction) are derived from the ECAR curve. (B) Representative kinetic study of glycolysis-dependent ECAR (mpH/min) in tolerogenic (TOL, green), LPS-tolerogenic (L-TOL, black), immature (IMM, red), and mature (MAT, blue) moDCs by using sequential addition of glucose (Gluc), oligomycin (Olig), and 2-DG. (C) Bars show basal ECAR levels (D) glycolytic rate (E) glycolytic capacity, and (F) glycolytic reserve of moDCs. The data were pooled from three independent experiments; mean 6 SEM. Statistical differences were analyzed by one-way ANOVA with a Tukey multiple comparison post-test. This figure has been modified from J Immunol 194 (11), 5174-5186, doi: 10.4049/jimmunol.1303316 (June 1, 2015). Reproduced and republished with copyright permission. Copyright 2015. The American Association of Immunologists, Inc. Please click here to view a larger version of this figure.
Discussion
This paper describes a method to generate from monocytes immature moDCs, tolerogenic moDCs and mature moDCs. The important steps in this protocol are discussed in detail in the following paragraphs. It is important to note that human peripheral blood is used as a starting material in this protocol and universal precautions for handling human blood should be practiced. Although it is technically feasible to derive DCs from bone marrow in humans 24, the in vitro DC differentiation from cells found in peripheral blood is preferred due to the availability of peripheral blood compared to bone marrow. Among the cells found in peripheral blood, hematopoietic CD34+ stem cells and monocytes are commonly used for the in vitro generation of DCs. Hematopoietic CD34+ stem cells are cultured with GM-CSF and TNF-α to derive CD1a+ and CD14+ subsets which are then further differentiated into Langerhans like cells and dendritic cells. Conversely, monocytes are cultured in GM-CSF and IL-4 to generate immature moDCs. Several protocols are used for the enrichment of monocytes from peripheral blood; for example, by adherence to plastic dishes, elutriation and isolation kits 25, 26. The advantages of the adherence protocol are minimum damage to cells and relatively cost effective however cell purity might be compromised; and an extra step is required to detach the cells for further experiments. Elutriation is a technique that separates cells based on their size and density. The advantages of elutriation are cell viability and monocytes can be readily used for further experiments; however this technique is limited by the availability of an elutriator and the inability to separate different populations of cells (T cells and monocytes) with similar sedimentation parameters. Commercially available isolation kits utilize magnetic microbeads to either positively select or negatively select the monocytic population. Some protocols are biased towards monocyte isolation using the negative selection as the isolated monocytes remain "untouched" (not bound by markers or microbeads). In this protocol, CD14 beads were used to positive select human monocytes from PBMCs. CD14 lacks a cytoplasmic domain and binding of antibody to CD14 does not trigger signal transduction. Moreover, the microbeads will detach from monocytes after culture and hence does not hinder the differentiation process. In addition, CD14 is strongly expressed on most monocytes and weakly on neutrophils and some myeloid dendritic cells, hence this method of isolation results in higher cell purity than the other methods17.
Blood monocytes can be differentiated into DCs or macrophages and the fate of monocytes greatly depends on the cytokine environment. In this paper, moDCs are generated by adding granulocyte-macrophage colony-stimulating factor (GM-CSF) and Interleukin 4 (IL-4) to human peripheral blood monocytes. GM-CSF is required for monocyte survival and IL-4 exerts an inhibitory activity on macrophage differentiation; and the combinatorial addition of GM-SCSF and IL-4 to monocytes yield a higher percentage of immature moDCs compared to individual cytokine27. There are other protocols that generate moDCs by adding tumor necrosis factor alpha (TNF-α), interferon alpha (IFN-α) and Interleukin 13 (IL-13) to peripheral blood monocytes 28,29,30. The combination of GM-CSF and IL-4 was optimized in the 1990s and now an accepted protocol that generates plastic immature DCs differentiated into immunogenic or tolerogenic moDCs and polarized into Th1, Th2 or Th17 promoting moDCs.
Immature moDCs are differentiated into tolerogenic moDCs by the addition of vitamin D3 and dexamethasone. There are several protocols to generate tolerogenic DCs for example, via nuclear factor-kappa B (NF-kB) inhibition, β-catenin activation, Vitamin D3, Dexamethasone and Rapamycin 31,32,33,34,35,9,36,37. Although both vitamin D3 and dexamethasone alone have been reported to induce a tolerogenic effect on DCs, the combination of vitamin D3 and dexamethasone result in a greater suppression of alloproliferation than when individual drugs are used. Therefore, the existing protocol for generation of tolerogenic DCs was modified to a combination of vitamin D3 and dexamethasone. This method is currently being accepted as a model for human tolerogenic DCs with a therapeutic utility. It is also important to note that the reconstituted Vitamin D3 and dexamethasone have a short shelf life.
In this protocol, Lipopolysaccharides (LPS) was added as a DC maturation inducer. Immature moDCs can also be induced to maturation using pro-inflammatory cocktail: (TNF-α), Interleukin 1 beta (IL-1β), Interleukin 6 (IL-6) and prostaglandin E2) or pro-inflammatory cytokines (TNF-α and interferon gamma (IFN-Γ)). Pro-inflammatory cocktail generates mature moDCs with high co-stimulatory and migratory functions but they produce relatively low levels of IL-1238. TNF-α or IFN-Γ alone is not able to induce a stable dendritic phenotype 39. LPS stimulates Toll-like receptor 4 (TLR4), mediates the activation of NF-kB and mitogen activated protein kinases (MAPKs) to induce DC maturation. DC maturation induced by LPS shows an up-regulation of DC maturation markers (CD83, CD86, HLA-DR) and also led to the production of IL-12p70. In addition, this step can be further modified to pair LPS with TLR3 agonists to produce mature DCs for clinical cancer vaccines. In this paper, tolerogenic DCs are shown to be resistant to maturation upon LPS treatment. These semi-mature like DCs are not immunogenic and do not release pro-inflammatory cytokines 40.
The limitations of this protocol lie in the differentiation process. The process takes 8 days from Day 0 to Day 7 which poses a difficulty to be adapted into high throughput analyses. A modification in the protocol is required to shorten the differentiation process yet yield high numbers of viable DCs in the different states. Secondly, the DCs are generated by addition of cytokines in this protocol and these cytokines do not sustain DC population for long period of time. Moreover, cytokines are used in concentrations much higher than in vivo and could result in biased development of pathways that are not physiologically identical to in vivo DCs. For example in vitro cultures of DC precursors have been shown to respond to GM-CSF, which is not an essential cytokine for normal DC differentiation in vivo41. Nevertheless, cytokine stimulation can be a useful method to generate high numbers of DC in vitro for experimentation. The ability to subject these cells generated from this protocol to other analyses such as immunofluorescence staining, flow cytometry, allo reaction studies and metabolic studies increases the usefulness of this method. These in vitro DCs serve as a good model to improve the knowledge of DC development, maturation and antigen presentation which is previously difficult to do with the rare numbers of in vivo DCs.
DCs' ability to regulate immunological immunity versus tolerance makes them attractive candidates in therapeutics against cancer and autoimmune diseases 42,43,44,45. Immunogenic DCs generated in this protocol can be used to improve vaccination efficacy against infectious diseases and tumors; while tolerogenic DCs can be used to control unwanted T cell responses and prevent rejections following transplantation. The intricate balance between immunity and tolerance depends immensely on DC differentiation status. DC differentiation is a coordinated cellular program that is governed by multiple signaling pathways and metabolic fate. Different differentiation states of DC differ in bioenergetic and biosynthesis needs; for example, activated DCs require more energetic metabolic adaptations important for survival and migration as compared to DCs at resting state. It is important to note that vitamin D3, dexamethasone and rapamycin are known for their ability to induce tolerogenic DCs, have been described to influence DC metabolism. In this paper, the energetic metabolism of moDCs from different differentiation states were characterized using extracellular flux analyzers and tolerogenic moDCs exhibited the highest metabolic plasticity and LPS-induced maturation decreased this plasticity. Anabolic metabolism supports DCs maturation while catabolic metabolism influences tolerogenic DC functions46. The DCs generated from this protocol can be used to assess whether altering the metabolic state of DCs hold the key to modifying immunity and tolerance in therapeutics. In conclusion, we presented a protocol for the generation of immature, tolerogenic and mature moDCs crucial for studying DCs' immunoregulatory functions.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by Agency for Science, Technology and Reasearch Core Funding (to J.E.C).
Materials
| Ficoll | GE Healthcare | 17-1440-03 | PBMC isolation |
| Syringe | Becton, Dickinson | 302832 | PBMC isolation |
| 1.5 mL centrifuge tube | Axygen | MCT-150-C | PBMC isolation |
| 15 mL falcon tube | Falcon | 352096 | PBMC isolation |
| 50 mL falcon tube | Falcon | 352070 | PBMC isolation |
| Centrifuge | Eppendorf | 5810R | PBMC isolation |
| 0.2 µm filter | Sartorius stedim biotech | 17597 | PBMC isolation |
| MACs kit | Miltenyi biotec | 130-042-201 | Monocyte enrichment |
| MiniMACS Separator | Miltenyi biotec | 130-042-102 | Monocyte enrichment |
| Cell culture grade water | Invitrogen, Life Technologies | Cell culture | |
| RPMI | Gibco, Life Technologies | 11875-093 | Cell culture |
| FBS | Hyclone | SH30070103 | Cell culture |
| Penicillin-streptomycin | Gibco, Life Technologies | 15140 | Cell culture |
| Phosphate Buffered Saline |
Gibco, Life Technologies | 10010-031 | Cell culture |
| NEAA | Gibco, Life Technologies | 11140-040 | Cell culture |
| EDTA | Gibco, Life Technologies | 15575 | Cell culture |
| HEPES | Gibco, Life Technologies | 15630-080 | Cell culture |
| Sodium Pyruvate | Gibco, Life Technologies | 11360-070 | Cell culture |
| GM-CSF | Miltenyi biotec | 130-093-868 | Cell culture |
| IL-4 | Miltenyi biotec | 130-093-924 | Cell culture |
| Vitamin D3 | Sigma | D1530 | Cell culture |
| Dexamethasone | Sigma | D2915 | Cell culture |
| LPS | Sigma | l2755 | Cell culture |
| trypan blue | Gibco, Life Technologies | 15250-061 | Cell culture |
| PerCP-conjugated HLADR | BioLegend | 307628 | Cytometry |
| PE-conjugated CD80 | BD Biosciences | 557227 | Cytometry |
| PE-conjugated CD83 | BD Biosciences | 556855 | Cytometry |
| PE-conjugated CD86 | BD Biosciences | 555665 | Cytometry |
| APC-conjugated CD11c | BD Biosciences | 340544 | Cytometry |
| PE-conjugated CD14 | Miltenyi biotec | 130-091-242 | Cytometry |
| PE-conjugated BDCA3 | Miltenyi biotec | 130-090-514 | Cytometry |
| APC-conjugated ILT3 | eBioscience | 12-5139-73 | Cytometry |
| Isotype matched PerCP- conjugated Mab |
BioLegend | 400250 | Cytometry |
| Isotype matched PE- conjugated Mab |
Miltenyi biotec | 130-091-835 | Cytometry |
| Isotype matched APC- conjugated Mab |
Miltenyi biotec | 130-091-836 | Cytometry |
| BD LSR II Flow Cytometer | BD Pharmingen | BD LSR II | Cytometry |
| cytofix/cytoperm | BD Biosciences | 554714 | Cytometry |
| APC/CY7-conjugated CD25 | BD pharmingen | 557753 | Cytometry |
| PE/CY7-conjugated CD4 | Biolegend | 300512 | Cytometry |
| PerCP-conjugated CD3 | Biolegend | 300428 | Cytometry |
| EasySep Human CD4+ T cell enrichment kit | STEMCELL Technologies | 19052 | Alloreaction study |
| EasySep magnet | STEMCELL Technologies | 18000 | Alloreaction study |
| Cell Trace CFSE cell proliferation kit | Molecular probes | C34554 | Alloreaction study |
| HBSS | Gibco, Life Technologies | 14025092 | Alloreaction study |
| Alexa Fluor 647-conjugated FoxP3 | BD Biosciences | 560889 | |
| Milliplex MAP Human Cytokine/Chemokine magnetic bead panel |
Millipore | HCYTOMAG-60K | Cytokine analysis |
| 5 mL Polystyrene tube | Falcon | 352058 | Cytokine analysis |
| Luminex Sheath Fluid | Millipore | SHEATHFLUID | Cytokine analysis |
| FLEXMAP 3D system with xPONENT software | Luminex Corporation | FLEXMAP 3D | Cytokine analysis |
| MitoTracker Red CMXRos | Cell Signalling | 9082 | Mitochondrial activity |
| DMSO | Sigma Aldrich | D2650 | Mitochondrial activity |
| XF Assay Medium (OCR) | Seahorse Bioscience | 102352-000 | Metabolic adaptation |
| Glucose | Sigma Aldrich | G8769 | Metabolic adaptation |
| XF Base Medium (ECAR) | Seahorse Bioscience | 102353-100 | Metabolic adaptation |
| L-glutamine | Gibco, Life Technologies | 25030-081 | Metabolic adaptation |
| Calibrant | Seahorse Bioscience | 100840-000 | Metabolic adaptation |
| XF Cell Mito Stress kit | Seahorse Bioscience | 103015-100 | Metabolic adaptation |
| XF Glycolysis Stress kit | Seahorse Bioscience | 103020-100 | Metabolic adaptation |
| Seahorse | Seahorse Bioscience | XFe96 | Metabolic adaptation |
Riferimenti
- Jolles, S. Paul Langerhans. J Clin Pathol. 55 (4), 243-24 (2002).
- Steinman, R. M., Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 137 (5), 1142-1162 (1973).
- Steinman, R. M., Swanson, J. The endocytic activity of dendritic cells. J Exp Med. 182 (2), 283-288 (1995).
- Hemmi, H., Akira, S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 86, 120-135 (2005).
- Mueller, D. L. Mechanisms maintaining peripheral tolerance. Nat Immunol. 11 (1), 21-27 (2010).
- Inaba, K., et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 188 (11), 2163-2173 (1998).
- Caux, C., et al. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J Exp Med. 180 (5), 1841-1847 (1994).
- Paavonen, J., Lehtinen, M. Interactions between human papillomavirus and other sexually transmitted agents in the etiology of cervical cancer. Curr Opin Infect Dis. 12 (1), 67-71 (1999).
- Ohtani, M., et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 112 (3), 635-643 (2008).
- Wilde, B., et al. Dendritic cells in renal biopsies of patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 24 (7), 2151-2156 (2009).
- Al-Hello, H., et al. An enterovirus strain isolated from diabetic child belongs to a genetic subcluster of echovirus 11, but is also neutralised with monotypic antisera to coxsackievirus A9. J Gen Virol. 89, 1949-1959 (2008).
- Ziegler-Heitbrock, L., et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 116 (16), 74-80 (2010).
- Osugi, Y., Vuckovic, S., Hart, D. N. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 100 (8), 2858-2866 (2002).
- Reynolds, G., Haniffa, M. Human and Mouse Mononuclear Phagocyte Networks: A Tale of Two Species. Front Immunol. 6, (2015).
- Jefford, M., et al. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood. 102 (5), 1753-1763 (2003).
- Menck, K., et al. Isolation of human monocytes by double gradient centrifugation and their differentiation to macrophages in teflon-coated cell culture bags. J Vis Exp. (91), (2014).
- Zhou, L., et al. Impact of human granulocyte and monocyte isolation procedures on functional studies. Clin Vaccine Immunol. 19 (7), 1065-1074 (2012).
- Menon, V., Thomas, R., Ghale, A. R., Reinhard, C., Pruszak, J. Flow cytometry protocols for surface and intracellular antigen analyses of neural cell types. J Vis Exp. (94), (2014).
- Faresjo, M. A useful guide for analysis of immune markers by fluorochrome (Luminex) technique. Methods Mol Biol. 1172, 87-96 (2014).
- Baker, H. N., Murphy, R., Lopez, E., Garcia, C. Conversion of a capture ELISA to a Luminex xMAP assay using a multiplex antibody screening method. J Vis Exp. (65), (2012).
- Defawe, O. D., et al. Optimization and qualification of a multiplex bead array to assess cytokine and chemokine production by vaccine-specific cells. J Immunol Methods. 382 (1-2), 117-128 (2012).
- Nicholls, D. G., et al. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. (46), (2010).
- Chunharas, A., Pabunruang, W., Hongeng, S. Congenital self-healing Langerhans cell histiocytosis with pulmonary involvement: spontaneous regression. J Med Assoc Thai. 85, 1309-1313 (2002).
- Bai, L., Feuerer, M., Beckhove, P., Umansky, V., Schirrmacher, V. Generation of dendritic cells from human bone marrow mononuclear cells: advantages for clinical application in comparison to peripheral blood monocyte derived cells. Int J Oncol. 20 (2), 247-253 (2002).
- Felzmann, T., et al. Monocyte enrichment from leukapharesis products for the generation of DCs by plastic adherence, or by positive or negative selection. Cytotherapy. 5 (5), 391-398 (2003).
- Berger, T. G., et al. Efficient elutriation of monocytes within a closed system (Elutra) for clinical-scale generation of dendritic cells. J Immunol Methods. 298 (1-2), 1-2 (2005).
- Sallusto, F., Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 179 (4), 1109-1118 (1994).
- Pace, S. T., Gelman, B. B., Wong, B. R. Primary Langerhans cell histiocytosis of the lacrimal gland in an adult. Can J Ophthalmol. 50 (3), 40-43 (2015).
- Ravanfar, P., Wallace, J. S., Pace, N. C. Diaper dermatitis: a review and update. Curr Opin Pediatr. 24 (4), 472-479 (2012).
- Gebhardt, C., et al. A case of cutaneous Rosai-Dorfman disease refractory to imatinib therapy. Arch Dermatol. 145 (5), 571-574 (2009).
- Vazquez, P., Robles, A. M., de Pablo, F., Hernandez-Sanchez, C. Non-neural tyrosine hydroxylase, via modulation of endocrine pancreatic precursors, is required for normal development of beta cells in the mouse pancreas. Diabetologia. 57 (11), 2339-2347 (2014).
- Pellacani, G., et al. Distinct melanoma types based on reflectance confocal microscopy. Exp Dermatol. 23 (6), 414-418 (2014).
- Shi, Y., et al. Hepatic involvement of Langerhans cell histiocytosis in children–imaging findings of computed tomography, magnetic resonance imaging and magnetic resonance cholangiopancreatography. Pediatr Radiol. 44 (6), 713-718 (2014).
- Haustein, M., Terai, N., Pablik, J., Pillunat, L. E., Sommer, F. Therapy-resistant swelling of the upper eyelid in childhood. Ophthalmologe. 111 (1), 53-57 (2014).
- Haidinger, M., et al. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J Immunol. 185 (7), 3919-3931 (2010).
- Macedo, C., Turquist, H., Metes, D., Thomson, A. W. Immunoregulatory properties of rapamycin-conditioned monocyte-derived dendritic cells and their role in transplantation. Transplant Res. 1 (1), 16 (2012).
- Fischer, R., Turnquist, H. R., Taner, T., Thomson, A. W. Use of rapamycin in the induction of tolerogenic dendritic cells. Handb Exp Pharmacol. 188 (188), 215-232 (2009).
- Nicolette, C. A., et al. Dendritic cells for active immunotherapy: optimizing design and manufacture in order to develop commercially and clinically viable products. Vaccine. 25, 47-60 (2007).
- Han, T. H., et al. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J Immunother. 32 (4), 399-407 (2009).
- Paananen, A., et al. Molecular and biological analysis of echovirus 9 strain isolated from a diabetic child. J Med Virol. 69 (4), 529-537 (2003).
- HC, O. N., Wilson, H. L. Limitations with in vitro production of dendritic cells using cytokines. J Leukoc Biol. 75 (4), 600-603 (2004).
- Mest, H. J., et al. Glucose-induced insulin secretion is potentiated by a new imidazoline compound. Naunyn Schmiedebergs Arch Pharmacol. 364 (1), 47-52 (2001).
- Puc, J., et al. Mitochondrial activity after cold preservation of pancreatic islet cells treated with pefloxacin (PFX). Ann Transplant. 3 (1), 38-41 (1998).
- Alarcon, C., Serna, J., Perez-Villamil, B., de Pablo, F. Synthesis and differentially regulated processing of proinsulin in developing chick pancreas, liver and neuroretina. FEBS Letters. 436 (3), 361-366 (1998).
- Jekunen, A. P., Kairemo, K. J., Paavonen, T. Imaging of Hand-Schuller-Christian syndrome by a monoclonal antibody. Clin Nucl Med. 22 (11), 771-774 (1997).
- Pearce, E. J., Everts, B. Dendritic cell metabolism. Nat Rev Immunol. 15 (1), 18-29 (2015).

