Enhancement of the Initial Growth Rate of Agricultural Plants by Using Static Magnetic Fields
Summary
The goal of this protocol is to demonstrate the acceleration of the initial growth rate of plants by applying static magnetic fields with no external energy.
Abstract
Electronic devices and high-voltage wires induce magnetic fields. A magnetic field of 1,300-2,500 Gauss (0.2 Tesla) was applied to Petri dishes containing seeds of Garden Balsam (Impatiens balsamina), Mizuna (Brassica rapa var. japonica), Komatsuna (Brassica rapa var. perviridis), and Mescluns (Lepidium sativum). We applied magnets under the culture dish. During the 4 days of application, we observed that the stem and root length increased. The group subjected to magnetic field treatment (n = 10) showed a 1.4 times faster rate of growth compared with the control group (n = 11) in a total of 8 days (p <0.0005). This rate is 20% higher than that reported in previous studies. The tubulin complex lines did not have connecting points, but connecting points occur upon the application of magnets. This shows complete difference from the control, which means abnormal arrangements. However, the exact cause remains unclear. These results of growth enhancement of applying magnets suggest that it is possible to enhance the growth rate, increase productivity, or control the speed of germination of plants by applying static magnetic fields. Also, magnetic fields can cause physiological changes in plant cells and can induce growth. Therefore, stimulation with a magnetic field can have possible effects that are similar to those of chemical fertilizers, which means that the use of fertilizers can be avoided.
Introduction
Germination is the growth of a plant that results in the formation of the seedling1. Under certain conditions, seed germination begins and the embryonic tissues resume growth. It begins with hydration to the seed in order to activate enzymes for germination. Seeds can be induced to germinate in vitro (in a Petri dish or test tube)1,2.
Static magnetic fields are special forces that cause movements of molecules with ionic charges by way of the Lorentz force3,4. Lorentz force is formed when an ionized or charged object moves under a magnetic field. Every material is formed with atoms which are composed of electrons and protons. When magnetic fields become present, whether it is static or alternating, it affects the movement of charged material. This also applies to plants and water molecules, which affects the intracellular molecule condition. In a previous study, electromagnetic coils were used to generate pulsed magnetic fields, and 'Komatsuna' plants were chosen as the subjects5. In the present study, magnet generated static magnetic fields were used to give a similar but different effects as an expansion study of Lorentz force.
The frequency of the magnetic field, rather than its polarity, is a crucial factor for plant germination. Previous studies have suggested that maximum germination rates were 20% higher than control when the frequency of the magnetic field was approximately 10 Hz. When the field was removed in a retrograde manner, the growth rate was impaired5. Static magnetic fields have a considerable effect on the initial growth6-8, primarily on germination6 and root growth7.
In the present study, we used static magnets to examine the possibility of regulating the growth of agricultural plants by using magnetic fields. In particular, we aimed to determine whether certain conditions of magnetic field application could increase the growth rates to higher levels than those mentioned in the literature. Furthermore, if the initial germination of plants can be successfully increased using a magnetic field, the use of chemical fertilizers can be avoided.
Protocol
1. Initial Settings
- Agricultural Plant Species
- Use Garden Balsam (Impatiens balsamina), Mizuna (Brassica rapa var. japonica), Komatsuna (Brassica rapa var. perviridis), and Mescluns (Lepidium sativum) seeds.
NOTE: Impatiens balsamina (Garden Balsam or Rose Balsam) is a species native to India; a few members are also located in Myanmar. Komatsuna (Brassica rapa var. perviridis or komatsuna) is a variant of the same species as the common turnip. Garden cress (Lepidium sativum) is a type of herb that is taxonomically related to watercress and mustard. They have similar flavors and scent, for which they are commercially utilized5,7.
- Use Garden Balsam (Impatiens balsamina), Mizuna (Brassica rapa var. japonica), Komatsuna (Brassica rapa var. perviridis), and Mescluns (Lepidium sativum) seeds.
- Plant Cultures
- Culture Garden Balsam (Impatiens balsamina), Mizuna (Brassica rapa var. japonica), Komatsuna (Brassica rapa var. perviridis), and Mescluns (Lepidium sativum) seeds in a 100 mm diameter (100 pi) Petri dish. Ensure that one plate contains only one type of species.
- For culture conditions, place the seeds on a cellulose towel. Immerse the towel and seeds in triple distilled water. Measure and confirm that the indoor lab RT is 18-25 °C, with humidity ranging 65-75% (please check section 3.1.2).
- For number of seeds, culture 10 ± 1 seeds of Garden Balsam, 50 ±10 seeds of Mizuna, 330 ± 20 seeds of Komatsuna, and 380 ± 20 seeds of Mescluns. Use identical conditions measured as 18-25 °C, with humidity ranging 65-75% (please check section 2.1.1).
NOTE: All experiments were performed on indoor conditions with the regulated humidity and temperature range in lab. The humidity and temperature was not static, but provided the identical conditions for magnet treated group and control.
2. Culture of Four Agricultural Plants
- Experimental Procedure
- Follow section 1.2.3) for species of plant and culture conditions in control and magnet applied group.
- Apply three magnets of 1,750 ± 350 Gauss (10,000 Gauss = 1 Tesla) at the bottom of the 100 pi dishes for Garden Balsam. During the application, ensure that the three magnets are not in direct contact with the seeds, and are separated by the plastic bottom of the petri dish. The direct distance between seeds and magnets should be 2-4 mm. Apply magnets for 168 hr (7 days) for four agricultural plants.
- Following all steps identically in 2.1.2), apply two magnets, one (facing N side upward) on the top and other magnet (facing S side upward) on the bottom of Garden Balsam culture plate.
NOTE: The poles are applied differently in Garden Balsam. However, the pole orientation is not considered as a crucial factor in this study for growth alterations, as all environments are identical except for the direction of the magnetic flux. The purpose of N and S pole application for Garden Balsam was to see its practical ability in using it in fields, where pole orientation could be hard to manage.
3. Tubulin Staining of Garden Balsam
- Magnet Applications with Regulated Light Condition
- Place two magnets (N pole facing up) bottom of the 100 mm plate for 48 hr, using conditions in step 1.2.2.
NOTE: For modification of light, the culture dishes were placed on a plastic shelf in the incubator. Incubator was used for interception of light and maintaining temperature at 25 °C for 48 hr in dark environments. Eventually, this condition was not used in this experiment due to high variations in growth length.
- Place two magnets (N pole facing up) bottom of the 100 mm plate for 48 hr, using conditions in step 1.2.2.
- Plant Staining
- Fix the entire impatiens SPP double flower plant, (including stem and roots) grown on identical conditions with step 3.1.2) in 4% paraformaldehyde and 0.1 M phosphate buffer (pH 7.4) for 15 min.
- Remove the impatiens sample and immerse for 2 hr in blocking buffer (2% horse serum/1% bovine serum albumin/0.1% Triton X-100 in PBS, pH 7.5). Wash the impatiens sample by immersing with PBS for 15 min.
- For double immunostaining, incubate the sample with primary antibody, anti-alpha tubulin (1:1,000), O/N at 4 °C.
- Remove the sample and immerse the samples once with PBS for 10 min to wash. Use FITC-conjugated anti-mouse IgG (1:400) as the secondary antibody and incubate for 2 hr at 25 °C.
- Immerse the sample in PBS and cover slip the whole sample in bottom of 24 well plate. Obtain images using a conventional fluorescence microscope to observe tubulin orientation (λ = 550 nm, magnified to 100X, 200X and 400X).
NOTE: In this case, the magnet treated group (n = 10) and controls (n = 11) were confirmed for Garden Balsam (Impatiens balsamina) only, grown in non-dark conditions.
4. Data Collection Methods
- Time-lapse Creation of Four Agricultural Plant Growth
- Photograph the plant at 10 min intervals, by setting shutter to auto (this can be done in any digital camera). Set the aperture to F 3.2 and the ISO value to 400.
- Collect 700-900 pictures for 7-10 days. Connect the camera with electric wires since battery could be depleted.
- Drag the pictures by clicking and dropping each picture chronologically under the streaming line with moviemaking software (see materials and equipment table). Put it on a streaming line in equal durations of 0.045-0.05 sec for each into a film total of 30-40 sec. Check so that there are no dark gaps with selecting the each picture in a chronological order.
- After step 4.1.3, click play button in software to ensure the compiled-movie into a 30-40 sec time-lapse video slide and click render and save to .mpeg or .avi format. For size markers, use Canadian Quarter, an American Penny, and a centimeter ruler on the side of the photo.
- Perform t-test and box plot for statistical analysis11,12.
NOTE: Groups of five-number summaries were used to calculate the lower limit (L) value as Q1 – [1.5 × (Q3 – Q1)] and the higher limit (H) value as Q3 + [1.5 × (Q3 – Q1)]. This approach was incorporated into step 1.2.2 for length data collection11. The L and H values show the 99% area of the T-distribution plots, which means that the data points observed outside this range can be considered outliers. Box plots and Student's t-test were used to analyze the differences in the heights of seedlings12.
Representative Results
Tubulin staining showed dispersed or thinned structures in plants grown in the presence of the magnet compared to the control (Figure 2). Moreover, 7 day time-lapse studies with agricultural plants including Komatsuna (Brassica rapa var. perviridis) and Mescluns (Lepidium sativum) indicated that a magnet derived static magnetic field increases the initial growth of these plants (Figure 3).
These results suggest that the group exposed to a magnetic field had a notable growth change (Figure 1). Plants grown in dark environments did not show any difference, suggesting that only the light existing condition was applicable in a 7 day time-lapse experiment. Three representative agricultural plants were used in this study, but more plants could have been used. Crops and other plants may be investigated using the same protocol. In previous studies, the growth rate increased by 20%, whereas the present results showed a 1.4-fold increase, which is 40%. Thus, application of a magnet with static magnetic field was more effective than the application of alternating magnetic pulses.
Determining the effect of a magnetic field can be complicated because any molecular structure with electric charge may be influenced3,4. The static magnetic field appeared to influence the initial growth rate of garden balsam in a cultured cellulose tower tissue. The value was statistically significant and was approximately 1.4 times the growth rate of the control. Tubulin is essential for maintaining plant structure during cell elongation and growth9.
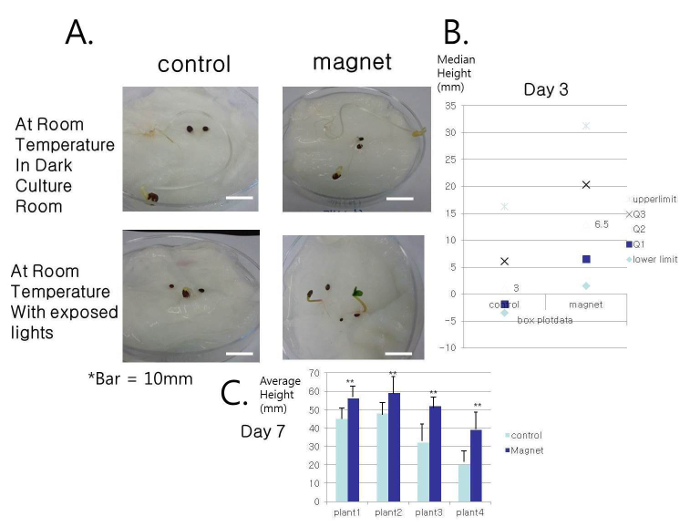
Figure 1. Growth of Garden Balsam. (A) The growth of Garden Balsam treated with a static magnetic field under dark conditions was marginal; however, the plants grew faster when exposed to light (the only representative picture shown). (B) When exposed to light, on day 3, the difference in height was statistically significant (p <0.01, two-sided t-test). (C) Each individual plant's height was higher until day 7 (**: upper bound of standard error for measurement). Dark conditions did not induce any differences, indicating that the effects of the magnetic field could be associated with hormones. Please click here to view a larger version of this figure.
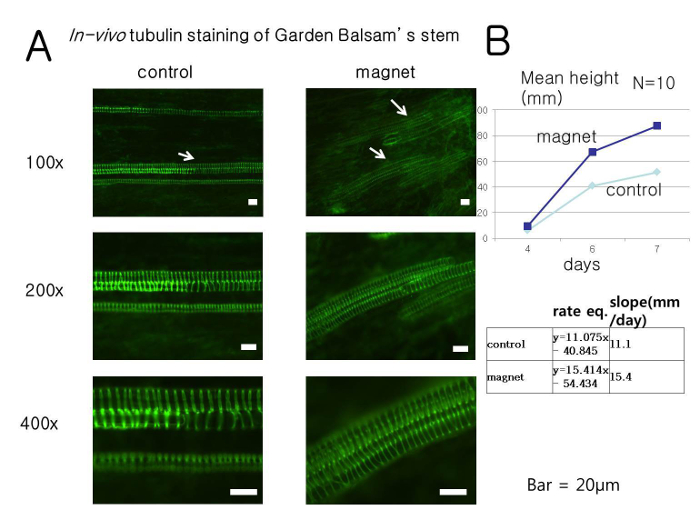
Figure 2. Tubulin staining of Garden Balsam and the increase in the growth rate of Garden Balsam after the application of a magnetic field. (A) Garden Balsam showed a dispersed distribution of tubulin structure when a magnetic field was applied. This finding indicates that growth-arresting protein structures like tubulin (and possibly actin) are affected by static magnetic fields. (B) The mean growth rate was 1.4-fold higher than that of the control, and the mean height was higher in the group treated with the magnetic field. Please click here to view a larger version of this figure.

Figure 3. A magnetic field facilitated the growth of Mescluns (Lepidium sativum, front) and Komatsuna (Brassica rapa var. perviridis). The seed supplemented petri dish was treated with a magnetic field of 1,750 ± 350 Gauss and observed for 7 days with a time-lapse interval of 10 min. The time-lapse video was cut to 15 fragments of 11 hr each. Please click here to view a larger version of this figure.

Supplementary Video 1: Growth timelapse of Garden Balsam (Impatiens balsamina). A Garden Balsam (Impatiens balsamina) seed supplemented Petri dish was treated with a magnetic field of 1,750 ± 350 Gauss and then observed for 7 days with a time-lapse interval of 10 min. The video was rearranged into a 30 min film. Please click here to view this video. (Right-click to download.)

Supplementary Video 2: Comparison of the growth of 3 plants grown in triplicate. Mizuna (Brassica rapa var. japonica), Komatsuna (Brassica rapa var. perviridis), and Mesclun (Lepidium sativum) seeds are shown in a 100 mm diameter (100 pi) Petri dish. Under identical conditions to those of Garden Balsam, the three species were evaluated separately, which revealed that the magnet's effect is broadly observed in agricultural plants. Please click here to view this video. (Right-click to download.)
Discussion
In all conditions, magnets should be applied under the petri dish. This study examined the influence of magnetic fields on growth rate of seeds for several agricultural species, with focus on Garden Balsam as a representative of agricultural plants. For instance, tubulin staining was performed on Garden Balsam to evaluate the molecular-level changes in root and stem skeletal micro-structures suggesting influence of the magnetic field in length proliferation. Both the N and S poles of the magnet were applied in a long-term (7-10 d) follow-up study using Garden Balsam. Three other species, Mizuna (Brassica rapa var. japonica), Komatsuna (Brassica rapa var. perviridis), and Mesclun (Lepidium sativum), were treated with N-pole oriented magnets. This was to further verify that the static magnetic field itself, not the poles, was a major factor in initial growth enhancement. Additionally, increasing the number of species provides support for broader applicability of magnet-derived initial growth facilitation in agricultural plants.
Many factors, such as nutrition, humidity, temperature, and light, might affect the rate of plant growth 3. Each of these was held constant across treatments. Nutritional supplement was excluded by only culturing in triple-distilled water. We first controlled for light-experiments were initially performed on Garden Balsam in an incubator under dark conditions. Growth patterns in the dark environment differed from those in the light environment. Therefore, we conducted subsequent experiments under light conditions (using identical amounts of light in all experimental groups). For the tubulin staining, the Garden Balsam was grown under controlled conditions (triple-distilled water, temperature 18-25°C, humidity 65%-75%). Other experiments of the 7-10-d follow-up study had identical "null conditions: no nutrition" conditions to those used with Garden Balsam (triple distilled water, temperature 18-25°C, humidity 65%-75%). With respect to magnet application, we used a strategy wherein we quantitatively increased the number of species and the duration of the magnet application to further investigate whether magnetic fields have a universal growth-facilitating effect on agricultural plants that is not limited to certain species. This idea was investigated using Garden Balsam (Impatiens balsamina), Mizuna (Brassica rapa var. japonica), Komatsuna (Brassica rapa var. perviridis) and Mescluns (Lepidium sativum).
The molecular basis of this phenomenon was partially clarified by the tubulin staining experiments9-11, but further investigation is needed for practical use. Precise magnetic application can be limited in humid environments owing to erosion of the magnet itself. The magnetic fields physically enhance the growth of agricultural plants. However, this does not prove that nutritional content also increases. Further analysis of the chemical contents of the plants should be conducted in order to determine whether the use of magnetic fields has an effect similar to that of a fertilizer. This could also be evaluated in environments in which nutrients are provided, as well as the nutrient-null conditions using distilled water that were employed in the present study. In addition to the quality (type, intensity, etc.) and quantity of applied magnets, cost can be another issue complicating such applications. It can be expensive to apply numerous magnets throughout an entire crop field.
Our findings suggest that static magnetic field application accelerates the germination rate and initial growth rate of several cultivated plant species. These findings demonstrate that a static magnetic field has a significant effect on plant growth, especially the germination 6 and root growth 7 of plants. Previous studies have suggested that maximum germination rates were 20% higher when the frequency of the magnetic field was approximately 10 Hz 5-6. During only 4 d of application of a magnetic field, the stem and root length increased. The group subjected to a magnetic field treatment (n = 10) showed a 1.4-times higher rate of growth than did the control group (n = 11) in a total of 8 d (p < 0.0005). This rate was 20% higher than that found in previous studies that used a pulsed magnetic field 6-9.
Given these findings, gene expression and regulation should also be studied in future experiments for clarification of the potential mechanisms behind the observed responses to magnetic fields10. Our findings suggest that the application of a magnetic field could increase crop growth rate, which could potentially help to address food and poverty issues worldwide. Moreover, the application of a magnetic field might be useful to reduce the use of chemical fertilizers.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study received supported from the National Research Foundation of Korea (NRF) (2011-0012728). A poster presenting this study was awarded the Best Poster Award by the Korean Society of Applied Biological Sciences (KSABC).
Materials
| Static magnets | JIM | N/A | 2000Gauss |
| 2% horse serum/1% bovine serum albumin/0.1% Triton X-100 | Sigma-Aldrich | Merged with 55514 | Blocking buffer |
| Primary antibody | Santa Cruz Biotechnology | sc-8035 | a-Tubulin |
| Secondary antibody | Santa Cruz Biotechnology | sc-2010 | FITC-conjugated anti-mouse IgG |
| time lapse photographic techniques | Manually controlled | N/A | ISO value 400 & aperture F 3.2 |
| Sony Vegas Pro 13.0 | Sony | N/A | N/A |
Riferimenti
- Martin, F. W. In vitro measurement of pollen tube growth inhibition. Plant Physiol. 49, 924-925 (1972).
- Pfahler, P. L. In vitro germination characteristics of maize pollen to detect biological activity of environmental pollutants. Environ Health Perspect. 37, 125-132 (1981).
- Yao, Z., Tan, X., Du, H., Luo, B., Liu, Z. A high-current microwave ion source with permanent magnet and its beam emittance measurement. Rev Sci Instrum. 79, 073304 (2008).
- Hendrickson, C. L., Drader, J. J., Laude, D. A., Guan, S., Marshall, A. G. Fourier transform ion cyclotron resonance mass spectrometry in a 20 T resistive magnet. Rapid Commun Mass Spectrom. 10, 1829-1832 (1996).
- Namba, K., Sasao, A., Shibusawa, S. EFFECT OF MAGNETIC FIELD ON GERMINATION AND PLANT GROWTH. Acta Hort. 399, 143-148 (1995).
- Hirota, N., Nakagawa, J., Kitazawa, K. Effects of a magnetic field on the germination of plants. Journals of Applied Physics. 85, 5717-5719 (1999).
- Penuelas, J., Llusia, J., Martinez, B., Fontcuberta, J. Diamagnetic Susceptibility and Root Growth Responses to Magnetic Fields in Lens culinaris, Glycine soja, and Triticum aestivum. Electromagnetic Biology and Medicine. 23, 97-112 (2004).
- Carbonell, M. V., Martinez, E., Amaya, J. M. Stimulation of germination in rice (Oryza Sativa L.) by a static magnetic field. Electro- and Magnetobiology. 19, 121-128 (2000).
- Oakley, R. V., Wang, Y. S., Ramakrishna, W., Harding, S. A., Tsai, C. J. Differential expansion and expression of alpha- and beta-tubulin gene families in Populus. Plant Physiol. 145, 961-973 (2007).
- Hoson, T., Matsumoto, S., Soga, K., Wakabayashi, K. Cortical microtubules are responsible for gravity resistance in plants. Plant Signal Behav. 5, 752-754 (2010).
- Kim, S., Im, W. Static magnetic fields inhibit proliferation and disperse subcellular localization of gamma complex protein3 in cultured C2C12 myoblast cells. Cell Biochem Biophys. 57, 1-8 (2010).
- Benjamini, Y. Opening the Box of a Boxplot. The American Statistician. 42, 257-262 (1988).

