Direct-current Stimulation and Multi-electrode Array Recording of Seizure-like Activity in Mice Brain Slice Preparation
Summary
Studies have shown that cathodal transcranial direct-current stimulation can produce suppressive effects on drug-resistant seizures. In this study, an in vitro experimental setup was devised in which the direct-current stimulation and multielectrode array recording of seizure-like activity were evaluated in mice brain slice preparation. The direct-current stimulation parameters were evaluated.
Abstract
Cathodal transcranial direct-current stimulation (tDCS) induces suppressive effects on drug-resistant seizures. To perform effective actions, the stimulation parameters (e.g., orientation, field strength, and stimulation duration) need to be examined in mice brain slice preparations. Testing and arranging the orientation of the electrode relative to the position of the mice brain slice are feasible. The present method preserves the thalamocingulate pathway to evaluate the effect of DCS on anterior cingulate cortex seizure-like activities. The results of the multichannel array recordings indicated that cathodal DCS significantly decreased the amplitude of the stimulation-evoked responses and duration of 4-aminopyridine and bicuculline-induced seizure-like activity. This study also found that cathodal DCS applications at 15 min caused long-term depression in the thalamocingulate pathway. The present study investigates the effects of DCS on thalamocingulate synaptic plasticity and acute seizure-like activities. The current procedure can test the optimal stimulation parameters including orientation, field strength, and stimulation duration in an in vitro mouse model. Also, the method can evaluate the effects of DCS on cortical seizure-like activities at both the cellular and network levels.
Introduction
Epilepsy is a common neurological disorder. Thirty percent of patients with epilepsy suffer from drug-resistant seizures1. Transcranial direct-current stimulation (tDCS) provides a noninvasive approach to control or alter network activities across large brain areas, such as seizures. Clinical studies have shown that tDCS effectively treats intractable seizures2 and can produce both short- and long-term suppressive effects on seizures3-5. However, the therapeutic mechanism of tDCS actions is still unclear. The brain slice model presented is an in vitro method to investigate how the therapeutic mechanism of tDCS actions alters the symptoms of seizure-like brain activities. Accordingly, to achieve its optimal effects, specific stimulation parameters including orientation, field strength, and stimulation duration need to be tested in an experimental model. Previous studies have shown that the orientation of the electric field is important to obtain therapeutic effects6. Thus, testing and arranging the orientation of electrodes relative to the position of the tested brain slice are feasible.
Frontal lobe epilepsy and anterior cingulate cortex (ACC) seizures are often drug-resistant7,8. Some studies have reported the application of tDCS in the cingulate cortex9-11. tDCS is shown to affect vigilance, decision making and emotion through alteration of ACC activities, and can modulate neuronal excitability and seizure activity in this brain region12. Therefore, suppressive effects of tDCS on ACC seizures might be helpful for clinical treatment and the evaluation of alternative treatments.
The present protocol describes the preparation of an electrode in the recording chamber for DCS of a brain slice and its effect on seizure-like activity recording with a multielectrode array (MEA).
Protocol
Procedures that involve animal subjects were approved by the Institutional Animal Care and Utilization Committee, Academia Sinica, Taipei, Taiwan.
1. Preparing Experimental Solution and Equipment for Multielectrode Array Recording
- Prepare artificial cerebral spinal fluid (aCSF; 124 mM NaCl, 4.4 mM KCl, 1 mM NaH2PO3, 2 mM MgSO4, 2 mM CaCl2, 25 mM NaHCO3, and 10 mM glucose, bubbled with 95% O2 and 5% CO2).
- Use two types of MEA probes: 6 x 10 planar MEA and 8 x 8 MEA. The former probe covers the region that comprises the cortex, striatum, and thalamus. The latter probe covers only the cortical region.
- Use a 60-channel amplifier with a band-pass filter set between 0.1 Hz and 3 kHz at 1,200 amplification. Acquire data at a 10 kHz sampling rate.
- Place two AgCl-coated silver wires inside the MEA chamber for DCS. Use the AgCl-coated silver wires to produce electric fields that are generated by an isolated stimulator.
- Place a tungsten electrode (diameter, 127 µm; length, 7.62 cm; 8° AC tapered tip; resistance, 5 MΩ) for thalamic stimulation, and place the reference electrode in the MEA chamber. Deliver the tungsten electrode's currents using an isolated stimulator that is controlled by a pulse generator.
2. Brain Slice Preparation
- Use male C57BL/6J mice, 4-8 weeks old. House the animals in an air-conditioned room (21-23 °C; 50% humidity; 12 hr/12 hr light /dark cycle, lights on at 8:00 AM) with free access to food and water.
- Take a 250 ml aliquot of the aCSF that was prepared in Step 1.1, and place it in a beaker that contains ice. At the same time, supply continuous gas that is composed of 95% O2 and 5% CO2.
- Surgery
- Anesthetize the animal with 4% isoflurane in a glass box for approximately 3 min. Once the animal reaches a surgical depth of anesthesia (indicated by the lack of a response to toe pinch), place it on a shallow tray that is filled with crushed ice, and remove the head using scissors.
- Expose the skull, and trim off the remaining muscle. Next, using rongeurs, peel away the dorsal surface of the skull from the brain. Trim away the sides of the skull using rongeurs. Sterilize all of the surgical instruments with a 75% ethanol solution.
- Using a spatula, cut the olfactory bulbs and nerve connections along the ventral surface of the brain, and remove the brain. After decapitation, quickly transfer the brain to a beaker filled with ice-cold oxygenated aCSF.
- Preparation of Medial Thalamus (MT)-ACC Brain Slice
Note: Prepare slices that contain the pathway from the MT to ACC13.- Hand-cut the brain block with two sagittal cuts 2.0 mm lateral to the midline in each hemisphere to display the subcortical anatomy. Then make two angled cuts. Make the first cross-cut parallel to the visible fiber tract in the striatum.
- Make the second cross-cut from the connection between the cerebellum and visual cortex to the midpoint between the anterior commissure and optic tract that are ventral and parallel to the thalamocingulate pathway.
- Attach the brain block to an angular plate (~120°) with cyanoacrylate adhesive, and make a cut just above the turning point of the pathway. Unfold the plate, flatten it, and glue it onto the chamber stage of a vibratome.
- Make medial thalamus-ACC brain slices (500 µm thick) and then immerse them in ice-cold oxygenated aCSF.Transfer slices to the recording chamber, and keep at 32 °C under continuous perfusion (12 ml/min) with oxygenated aCSF for 1 hr.
3. Preparation of Perfusion Chamber for Multielectrode Array Recording
- Preparation of Perfusion Chamber
- Place a MEA probe on a multi-channel system, and use two separate polyethylene tubes to connect the probe to a peristaltic pump. Use one tube to guide the aCSF into the MEA chamber and the other tube to guide the aCSF out of the chamber. Finally, continuously perfuse the preparation with warm (29-30 °C) oxygenated aCSF (8 ml/min).
- Transfer brain slice to MEA. Hold down the brain slice on the MEA using a wet cotton swab. Carefully move the brain slice to ensure the ACC is oriented above the electrodes.
- Use slice anchor kits and hold-downs to press the brain slice. This step ensures a good electrical connection between the slice and electrodes.
4. Generation of Electric Fields by DCS
Note: The definition of the electric field orientation was based on the direction of the axodendritic axis in the ACC. The orientations of dendrite and soma compartments were confirmed using Golgi staining12.
- Place the AgCl electrode (defined as the anode) proximal to the ACC, and place the other electrode (defined as the cathode) distal to the ACC. Record the field strength that is generated by the two field orientations (parallel and perpendicular to the ACC axodendritic fibers) by the MEA, and deliver the currents of the electric fields using a stimulator.
- Fix the distance of the AgCl electrodes (about 1.5-2 cm), and adjust the stimulator's current strength to make the DCS between 0.5 and 2 mA.
5. Electrically-induced Cortical Synaptic Responses
Note: Induce synaptic responses in the ACC by electrical stimulation in the MT, in which a programmable electrical stimulus generator produces rectangular biphasic current pulses.
- Repeat Section 3 above.
- Place a tungsten electrode in the MT, and deliver pulses from the stimulator to the thalamic region of the slices via bipolar tungsten electrodes.
- Use various current intensities to determine the threshold that elicits an ACC response. Here, use an intensity of ±150 µA and duration of 200 µsec, which elicited an 80% maximal response in the ACC in most slices.
- Move the tungsten electrode along the thalamocingulate pathway (from MT to corpus callosum) in the MT-ACC slice to obtain the optimal response profiles.
- Make 10-20 sweeps of ACC responses, and use the software to automatically average all of the ACC evoked by MT stimulation. The result iss the synaptic responses in ACC induced from MT stimulation by MT-ACC pathway.
6. Electrically-induced Seizure-like Activity
Note: Seizure-like activity was induced by the application of 4-aminopyridine (4-AP; 250 µM) and bicuculline (5 µM). Previous time-control studies showed that maximal and stable responses appeared 2-3 hr after drug application14.
- Repeat Section 5 above.
- Add drugs to the perfusion solution. Use 4-AP (250 µM) and bicuculline (5 µM). Mix the drugs uniformly, and continue perfusion for 2-3 hr.
- To facilitate seizure-like activity, maintain the perfusion pump at a relatively fast perfusion rate (8 ml/min), which can also help prevent the build-up of a pH gradient.
- Place a tungsten electrode in the MT, and deliver electrical stimulation (150 µA, 200 µsec duration) to obtain ACC response profiles.
- Make 10-20 sweeps and average the responses.
- Replace the perfusion solution with fresh aCSF to wash out the drugs. Repeat Step 6.5.
7. Testing Effect of DCS on Evoked Cortical Responses
- Repeat Sections 3 and 4. Ensure that uniform electric fields are generated by passing currents between two parallel AgCl-coated silver wires that are placed inside the MEA chamber. If there are no issues, the DCS stays between 0.5 and 2 mA.
- Turn off the DCS, and place a tungsten electrode to stimulate the thalamus (±150 µA, 200 µsec duration). To obtain maximal synaptic responses in the ACC, make 10-20 sweeps and average the responses.
- Simultaneously turn on the DCS (2 mV/mm DCS strength) and thalamic stimulation (350 µA, 200 µsec duration). Evaluate the changes of amplitude of the thalamic stimulation-evoked ACC response during DCS.
- Turn off the DCS, and add 4-AP (250 µM) and bicuculline (5 µM) to the perfusion solution. Then wait 2-3 hr. When the drugs affect the brain slice, the slice produces cortical seizure responses.
- Make 10-20 sweeps of ACC responses, and then measure the amplitude and duration of electrical evoked cortical seizure responses.
- After step 7.5, simultaneously turn on the DCS (2 mV/mm DCS strength) and thalamic stimulation (150 µA, 200 duration µsec). Evaluate changes in the amplitude and duration of evoked cortical seizure responses during DCS application.
- Replace the perfusion solution with fresh aCSF to wash out the drugs, and repeat steps 7.2 and 7.3.
- Collect all of the recording data, and group the data into the different experimental conditions. Evaluate the amplitude and duration of cortical seizure responses under different experimental conditions.
8. Data Analysis
- Use software (e.g., MC Rack software) to automatically average the recorded responses, and export the raw data to a spreadsheet. Analyze the amplitude and duration of the raw data and generate color figures.
- To detect oscillatory seizure events, use software to measure the baseline value and standard deviations (SD). Set 3 SD of the noise level as the threshold. Amplitudes of the peaks during an oscillation event that surpass this threshold are automatically detected.
- Perform the statistical analysis using Student's t-test.
- Express measurements and one-way analysis of variance (ANOVA) results in the text as mean ± SE, with n indicating the number of slices studied12.
Representative Results
Preparation of the Thalamocingulate Slice and MEA Recording System Setup
The MT-ACC slice from mice is a special slice preparation that allows exploration of the electrophysiological properties of the thalamocingulate pathway. Figure 1A shows the way in which the MT-ACC slice was prepared. The brain of the mouse was quickly removed and kept in cool oxygenated aCSF (Figure 1A, a, b). To reveal subcortical anatomy, the brain was cut 2.0 mm lateral from the midline in each hemisphere to acquire sagittal brain blocks (Figure 1A, c, d). Two angled ventral cuts were made in the brain blocks to retain the thalamocingulate pathway. The first cross-cut was made in the front of the brain block, parallel to the visible fiber tract in the striatum. The second cross-cut was made at the rear of the brain block from the connection between the cerebellum and visual cortex to the midpoint between the anterior commissure and optic tract. After the cuts were made, the brain block was glued on an angled plastic plate (120° angle), and a cut was made on the dorsal side directly through the cortex (Figure 1A, e-g). The brain block plate was glued to a vibratome and submerged in cool oxygenated aCSF. Finally, a few slices (500 µm thick) were taken from the brain block and incubated in oxygenated aCSF (Figure 1A, h, i).
Figure 1B shows the MEA recording system setup. The schematic diagram shows the perfusion and MEA systems connected to a recording computer. An empty MEA probe was placed inside the amplifier, and perfusion began at a flow rate of 8 ml/min. The MT-ACC brain slice was placed on the MEA probe while ensuring that the recording area was as close as possible to the center. A stimulator was used to stimulate the brain slice and generate the electric field. After all of the steps were completed, the system recorded the electrophysiological properties of the thalamocingulate pathway.
Figure 1C shows the recording area diagram and the way in which the MT-ACC brain slice was placed on the MEA probe. Figure 1C-a shows the appearance of the MEA probe. The black line (red arrow) on the probe helped the user determine the proper direction of the probe inside the amplifier. Figure 1C-b shows the major circuit of the MEA probe. Figure 1C-c shows the electrical array that was overlaid on the cortical tissue.
Testing Evoked Responses
To confirm preservation of the thalamocingulate pathway in the slice preparation, this study stimulated the thalamus and recorded at the ACC in the electrophysiological experiments. Only slices with postsynaptic potential in the ACC while delivering a small amount of current in the MT were used in the experiment. Figure 2A shows the positions of the stimulation and recording electrodes and typical thalamic stimulation-evoked responses in the ACC. To induce seizure-like activity, 4-AP (250 µM) and bicuculline (5 µM) were used to induce epileptiform activity. Typical 4-AP with bicuculline-induced spontaneous seizure-like activity was composed of an ictal onset, followed by a tonic phase and long duration. The traces were selected for magnification in Figure 2B. This study also attempted to deliver stimulation in the MT after inducing drug-induced seizures. 4-Aminopyridine/bicuculline-evoked epileptiform activity was induced after electrical stimulation (Figure 2C).
Testing Orientation of DCS and Slice
Previous clinical studies showed that the orientation of the electric field of cathodal DCS affects thalamic stimulation-evoked activity. Figure 3A shows the different orientations of the electric field, which was arranged parallel or perpendicular to the orientation of dendrite and soma compartments in the ACC. When the electric field was arranged parallel to neuronal cells, cathodal stimulation suppressed thalamic stimulation-evoked responses in the medial part of the ACC (Figure 3B, upper panel). When the electric field was arranged perpendicular to neuronal cells, no significant effects on thalamic stimulation-evoked responses were observed (Figure 3B, lower panel). Parallel cathodal DCS also suppressed 4-AP- and bicuculline-induced seizure-like activity in the medial part of the ACC (Figure 3C, upper panel). It shortened the duration of seizure-like activity, and no significant effect of perpendicular cathodal DCS was observed (Figure 3C, lower panel). These results confirmed that the orientation of the electric field was important in regulating synaptic transmission in the thalamocingulate pathway.
Effect of DCS on Seizure Activity
The clinical application of transcranial magnetic stimulation, tDCS, and DCS provides a noninvasive approach for the treatment of drug-resistant seizures. Previous studies showed that field stimulation modulated synaptic plasticity and influenced epileptiform activity in various brain regions. The present results showed that cathodal tDCS depressed thalamocingulate synaptic transmission. The amplitude of the stimulation-evoked responses and duration of seizure-like activity were depressed (9 of 11 slices, 81.82%; Figure 4A, left panel). Figure 4A (right panel) shows that 15 min of cathodal DCS effectively induced long-term depression (LTD) in the MT-ACC pathway and depressed evoked responses (N = 11, p <0.05). Figure 4B (left panel) shows that thalamic stimulation evoked robust seizure-like activity in the cingulate cortex. Thirty min after 15 min cathodal DCS application, the duration of thalamic stimulation-evoked seizure-like activity was shortened. The results also showed that the seizure duration was significantly decreased after 15 min cathodal DCS compared with no DCS application (N = 9, p <0.05; Figure 4B, right panel).
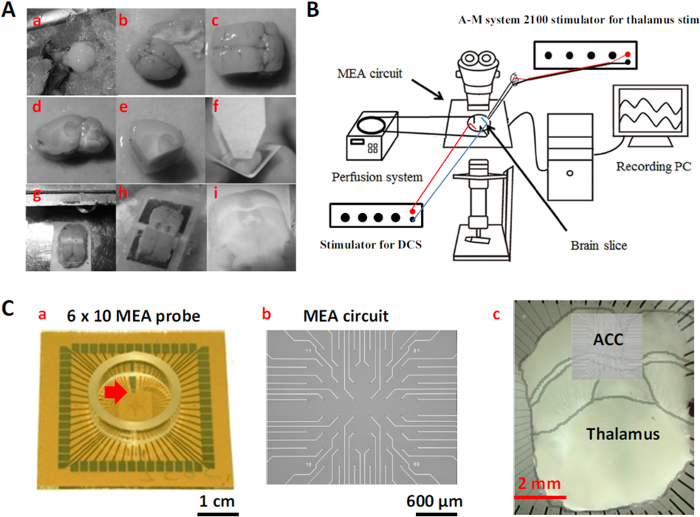
Figure 1: Preparation of the Thalamocingulate Slice and MEA Recording System Setup. (A) MT-ACC slice procedure (a) Remove the brain and (b) transfer to cool oxygenated aCSF. (c) Make two parasagittal cuts from the midline. (d) Side view of the medial part of the brain block. (e) Make two angled ventral cuts of the brain block. (f) Glue the brain block onto an angled plastic plate. (g) Make a dorsal cut and unfold the brain block. (h) Glue the brain block in a vibratome. (i) Collect slices from the brain block. (B) MEA recording system setup. (C) MEA recording area. (a) MEA probe. (b) MEA circuit. (c) The ACC of the brain slice was oriented above the electrode probes. Please click here to view a larger version of this figure.
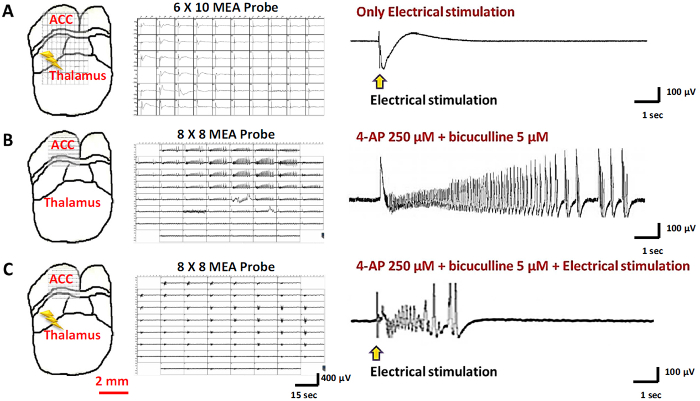
Figure 2: Different Evoked Responses to Thalamic Stimulation and Drug-induced Stimulation. (A) Thalamic stimulation-evoked responses in the ACC. (B) 4-Aminopyridine- and bicuculline-induced seizure-like activity. (C) Thalamic stimulation- and drug-induced seizure-like activity. Please click here to view a larger version of this figure.
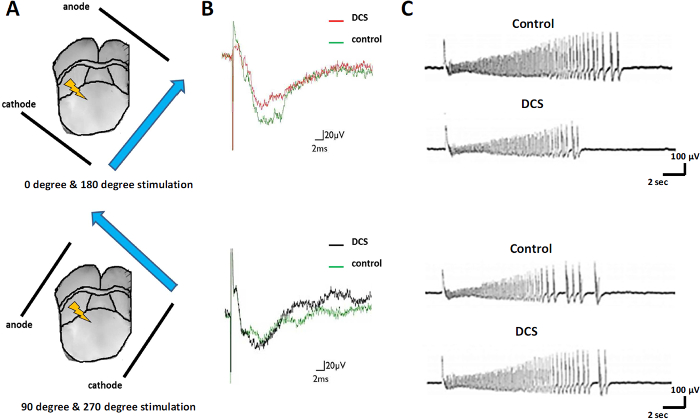
Figure 3: Effect of Different Orientations of DCS. (A) Different orientations of the electric field. (B) Thalamic stimulation-evoked responses with DCS. (C) Effect of cathodal DCS on seizure-like activity. Please click here to view a larger version of this figure.
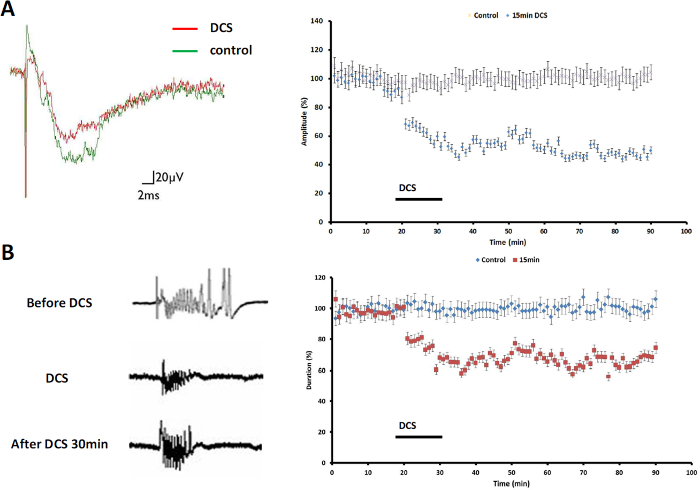
Figure 4: Effect of Cathodal tDCS on Seizure Activity. (A) Fifteen min cathodal DCS-induced LTD and depressed evoked activity. (B) The occurrence of seizure-like activity decreased during cathodal stimulation. The suppression of seizure-like activity by 15 min cathodal DCS endured even when the application of DCS was terminated. Please click here to view a larger version of this figure.
Discussion
In the present study, the effects of the duration and orientation of DCS on ACC seizure-like activity were tested. To obtain stable data in mouse brain slices, how to keep the integrity of the MT-ACC pathway and to avoid damage it is key, especially the steps in which two angled ventral cuts and a dorsal cut of the cortex are made. Moreover, the time to prepare the brain slice can also affect the activity of the brain slice, which should be the shortest time possible to keep the brain fresh and strong. A previous study showed that electrochemical damage to targeted tissue can occur in an in vivo preparation15. An in vitro brain slice preparation can be used to avoid this problem. In an in vitro preparation, the tissue does not directly contact the electrode, thus minimizing electrochemical effects16. The effects of DCS were compared with electrodes that were oriented in different directions. When the electrodes were oriented at 90° and 270°, DCS did not affect evoked activity (Figure 3). Thus, this controlled experiment excluded the possibility of any side effects of electrochemical reactions to DCS that damaged the tissue in our study. The protective recovery method of the brain slices is another key; the aCSF formula in this study provides an alternative to the protective cutting method and is highly effective for preservation of neurons in brain slices from 4 to 8 week-old animals. The method is not designed for use with animals of all ages; the use of Tris aCSF appears to be effective in young mice as is the use of the NMDG protective recovery method in mice aged 6 weeks and older. Therefore, users should keep in mind the relative age equivalencies across species to choose the best method for experiment.
Using a MEA to record brain slices is a common technique, but combining an electric field with a MEA recording system is not generally done. Affecting a DC field in conductive solution of the MEA recording system is an interesting approach, especially for periods of many seconds to minutes. A previous study showed that DCS application did not change the pH in the aCSF solution, indicating that the pH of the conductive solution was stable in this experimental setup12. A relatively fast perfusion rate (8 ml/min) was maintained to facilitate seizure-like activity, and any products of the chemical change in the MEA probe were washed out by the perfusion, thus avoiding the build up of a pH gradient. Multielectrode array recording technology is often limited by the type of brain slice and range of the recording electrode. The type of brain slice determines which circuit pathway is recorded, and the range of the recording electrode determines whether single or multiple brain nuclei are recorded. These conditions must be confirmed before the experiment.
Previous studies showed that the long-term effects of DCS occur through the modulation of synaptic transmission17. In the present study, cathodal DCS caused LTD in the MT-ACC pathway. The LTD or depotentiation of seizure-related potentiation was proposed to be part of the underlying mechanism of seizure suppression, suggesting that enhancement of the outcome of tDCS treatment may be possible. However, no published study has focused on the field strength at the cingulate cortex. The deep location of the cingulate cortex in the medial part of the cortex is difficult to test. For example, it is unavoidable that the current flow may affect the tissues and vessels that are closer to the surface. The difficulty of targeting deep tissue by tDCS may limit the application of tDCS for in vivo study. Therefore, to understand how DCS affects neuronal activities, a brain slice preparation should be used, as non-specific vascular effects need to be excluded.
For the purpose of establishing an experimental model, the described seizure is induced in a healthy brain. The seizure-like activities were further induced by a electrical pulse. The timing of the seizure occurrence could be precisely controlled when the DCS was applied. The results may provide more information for tDCS treatment. Another notable finding was the long-lasting changes in regional cortical excitability that were induced by tDCS. In the future, if the underlying mechanism of tDCS can be elucidated, then the combination of DCS and pharmacological therapy to enhance LTD in the treatment of epilepsy may be a very interesting development.
In conclusion, a methodology for investigating the effects of DCS on thalamocingulate and transcallosal synaptic plasticity and acute seizures was provided. The long-term effects of DCS on seizure-like activity in our brain slice preparation occurred through an LTD-like mechanism.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We are grateful for the technical support from the Neural Circuit Electrophysiology Core at Academia Sinica. This work was supported by the National Science Council (102-2320-B-001-026-MY3 and 100-2311-B-001-003-MY3) and Neuroscience Program of Academia Sinica.
Materials
| Anesthetic: | |||
| Isoflurane | Halocarbon Products Corporation | NDC 12164-002-25 | 4% |
| Name | Company | Catalog Number | Comments |
| aCSF (total:1L): | |||
| D(+)-Glucose | MERCK | 1.08337.1000 | 10 mM |
| Sodium hydrogen carbonate | MERCK | 1.06329.0500 | 25 mM |
| Sodium chloride | MERCK | 1.06404.1000 | 124 mM |
| (+)-Sodium L-ascorbate, >=98% | SIGMA | A4034-100G | 0.15 g / 2 c.c |
| Magnesium sulfate, anhydrous,ReagentPlus | SIGMA | M7506-500G | 2 mM |
| Calcium chloride dihydrate | MERCK | 1.02382.1000 | 2 mM |
| Sodium dihydrogen phosphate monohydrate | MERCK | 1.06346.1000 | 1 mM |
| Potassium chloride | May & Baker LTD Dagenham England | MS 7616 | 4.4 mM |
| Name | Company | Catalog Number | Comments |
| Drugs: | |||
| (+)-Bicuculline | TOCRIS | 0130 | 5 µM in aCSF |
| 4-Aminopyridine | TOCRIS | 0940 | 250 µM in aCSF |
| Name | Company | Catalog Number | Comments |
| Brain slice Preparation: | |||
| Vibratome | Vibratome | Series 1000 | Block slicing into 500 µm thick slices |
| Name | Company | Catalog Number | Comments |
| MEA system: | |||
| Multielectrode array (MEA) probes: 6 x 10 planar MEA | Multi Channel Systems | 60MEA500/30iR-Ti-pr MEAS 6×10 | electrode diameter, 30 µm; electrode spacing, 500 µm; impedance, 50 kΩ at 200 Hz |
| Multielectrode array (MEA) probes: 8 x 8 MEA | Ayanda Biosystems | 60MEA200/10iR-Ti-pr MEAS 8×8 | pyramidal-shaped electrode; diameter, 40 µm; tip height, 50 µm; electrode spacing, 200 µm; impedance, 1000 kΩ at 200 Hz |
| A 60-channel amplifier was used with a band-pass filter set between 0.1 Hz and 3 KHz at 1200X amplification | Multi-Channel Systems | MEA-1060-BC | |
| MC Rack software at a 10 KHz sampling rate | Multi-Channel Systems | Software for data collect and recordings | |
| control of a pulse generator | Multi-Channel Systems | STG 1002 | |
| slice anchor kits and hold-downs | Warner Instruments | SHD-26H/10; WI64-0250 | |
| Peristaltic Pump-minipuls3 | Gilsom | MINIPULS3 | perfusion rate : 8 ml/min |
| Name | Company | Catalog Number | Comments |
| Stimulation system: | |||
| Isolated stimulator | A-M Systems | Model 2100 | intensity of ±350 μA , duration of 200 μs |
| Tungsten electrode | A-M Systems | 575300 | placed in thalamus |
Riferimenti
- Schiller, Y., Najjar, Y. Quantifying the response to antiepileptic drugs: effect of past treatment history. Neurology. 70 (1), 54-65 (2008).
- Fregni, F., et al. A controlled clinical trial of cathodal DC polarization in patients with refractory epilepsy. Epilepsia. 47 (2), 335-342 (2006).
- Auvichayapat, N., et al. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul. 6 (4), 696-700 (2013).
- Chung, M. G., Lo, W. D. Noninvasive brain stimulation: the potential for use in the rehabilitation of pediatric acquired brain injury. Arch Phys Med Rehabil. 96 (4 Suppl), S129-S137 (2015).
- Del Felice, A., Magalini, A., Masiero, S. Slow-oscillatory Transcranial Direct Current Stimulation Modulates Memory in Temporal Lobe Epilepsy by Altering Sleep Spindle Generators: A Possible Rehabilitation Tool. Brain Stimul. 8 (3), 567-573 (2015).
- Garnett, E. O., Malyutina, S., Datta, A., den Ouden, D. B. On the Use of the Terms Anodal and Cathodal in High-Definition Transcranial Direct Current Stimulation: A Technical Note. Neuromodulation. , (2015).
- Biraben, A., et al. Fear as the main feature of epileptic seizures. J. Neurol. Neurosurg. Psychiatry. 70 (2), 186-191 (2001).
- Zaatreh, M. M., et al. Frontal lobe tumoral epilepsy: clinical, neurophysiologic features and predictors of surgical outcome. Epilepsia. 43 (7), 727-733 (2002).
- Karim, A. A., et al. The truth about lying: inhibition of the anterior prefrontal cortex improves deceptive behavior. Cereb. Cortex. 20 (1), 205-213 (2010).
- Keeser, D., et al. Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. J. Neurosci. 31 (43), 15284-15293 (2011).
- Nelson, J. T., McKinley, R. A., Golob, E. J., Warm, J. S., Parasuraman, R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS). Neuroimage. 85 (Pt 3), 909-917 (2014).
- Chang, W. P., Lu, H. C., Shyu, B. C. Treatment with direct-current stimulation against cingulate seizure-like activity induced by 4-aminopyridine and bicuculline in an in vitro mouse model. Exp. Neurol. 265, 180-192 (2015).
- Lee, C. M., Chang, W. C., Chang, K. B., Shyu, B. C. Synaptic organization and input-specific short-term plasticity in anterior cingulate cortical neurons with intact thalamic inputs. Eur. J. Neurosci. 25 (9), 2847-2861 (2007).
- Chang, W. P., Shyu, B. C., Pandalai, S. G. Involvement of the thalamocingulate pathway in the regulation of cortical seizure activity. Recent Research Developments in Neuroscience. 4, 1-27 (2013).
- Brummer, S. B., Turner, M. J. Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes. IEEE Trans. Biomed. Eng. 24 (1), 59-63 (1977).
- Durand, D. M., Bikson, M. Suppression and control of epileptiform activity by electrical stimulation: a review. Proc. IEEE. 89 (7), 1065-1082 (2001).
- Fritsch, B., et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 66 (2), 198-204 (2010).

