Murine Spinotrapezius Model to Assess the Impact of Arteriolar Ligation on Microvascular Function and Remodeling
Summary
We demonstrate a novel arterial ligation model in murine spinotrapezius muscle, including a step-by-step procedure and description of required instrumentation. We describe the surgery and relevant outcome measurements relating to vascular network remodeling and functional vasodilation using intravital and confocal microscopy.
Abstract
The murine spinotrapezius is a thin, superficial skeletal support muscle that extends from T3 to L4, and is easily accessible via dorsal skin incision. Its unique anatomy makes the spinotrapezius useful for investigation of ischemic injury and subsequent microvascular remodeling. Here, we demonstrate an arteriolar ligation model in the murine spinotrapezius muscle that was developed by our research team and previously published1-3. For certain vulnerable mouse strains, such as the Balb/c mouse, this ligation surgery reliably creates skeletal muscle ischemia and serves as a platform for investigating therapies that stimulate revascularization. Methods of assessment are also demonstrated, including the use of intravital and confocal microscopy. The spinotrapezius is well suited to such imaging studies due to its accessibility (superficial dorsal anatomy) and relative thinness (60-200 μm). The spinotrapezius muscle can be mounted en face, facilitating imaging of whole-muscle microvascular networks without histological sectioning. We describe the use of intravital microscopy to acquire metrics following a functional vasodilation procedure; specifically, the increase in arterilar diameter as a result of muscle contraction. We also demonstrate the procedures for harvesting and fixing the tissues, a necessary precursor to immunostaining studies and the use of confocal microscopy.
Introduction
Animal models of chronic ischemia are valuable tools for investigating the pathophysiology of ischemic diseases, such as peripheral artery disease, coronary artery disease, and cerebrovascular disease. In rodents, as in humans, arterial occlusion leads to structural remodeling of the vascular network, including arteriogenesis and angiogenesis. In healthy and younger patients, this remodeling can be sufficient to rescue tissue from ischemia-induced injury, but co-morbidities such as diabetes can severely compromise remodeling and recovery. Understanding the mechanisms underlying vascular remodeling events is essential for developing therapies that stimulate these endogenous revascularization processes.
Currently, femoral artery ligation or resection in the hindlimb is the standard technique for studying chronic ischemia-induced vascular remodeling in small animals4,5. Analysis of the diameter, connectivity, and reactivity of the microvessels that make up the vascular network downstream of the ligated femoral artery is difficult, however, due to the thickness of the muscles. We have developed an arteriolar ligation model in the mouse spinotrapezius muscle: unilateral ligation of the lateral feed artery in this stabilizing dorsal muscle1. The relatively thin spinotrapezius (60-200 μm) is amenable to en face immunostaining for the assessment of whole network topology with single-cell resolution, allowing a detailed examination of vascular remodeling events across the entire tissue. The spinotrapezius is also superficial and accessible, and thus its vessels are readily observable by intravital microscopy, for efficient characterization of the impact of remodeling and arteriolar ligation on vascular reactivity.
In this report, we describe in detail and demonstrate the mouse spinotrapezius arteriolar ligation model. Both in vivo and ex vivo methods of assessment following surgery are described, including measurement of functional vasodilation, which has been shown to be impaired under ischemic conditions6, and immunofluorescent imaging of whole-muscle microvascular networks. We also include results of two separate pilot studies to demonstrate the utility of the model. First, we utilized the arterial ligation model to induce a statistically significant increase in vessel tortuosity in C57BL/6 mice (Figure 2B). Tortuosity increases during arteriogenesis in arteriole collaterals. In other mouse strains, that are more vulnerable to ischemia due to the absence of collateral arterioles (e.g. Balb/c), capillary arterialization is observed10. Capillary arterialization is detected by increased diameters and the development of α-smooth muscle actin reactivity. Second, functional electrical stimulation of the muscle leads to vasodilation in terminal arterioles of the spinotrapezius (Figure 3B).
Protocol
1. Spinotrapezius Feed Artery Ligation Surgery
- Anesthetize mouse by intraperitoneal injection. In a 0.5 ml syringe, formulate:
0.06 ml of 100 mg/ml Ketamine
0.03 ml of 20.0 mg/ml Xylazine
0.01 ml of 0.4 mg/ml Atropine
0.30 ml of 0.9% Saline.
Dose: 0.01 ml* per gram body weight, i.p.
*Dose variable upon mouse strain and stock, adjust accordingly so mouse recovery is within an hour from induction.
- Apply standard ophthalmic ointment to prevent corneal desiccation.
- Remove hair from the back using trimming clippers, followed by brief application of depilatory cream.
- Disinfect the surgical field with chlorhexidine or povidone-iodine. Alternate povidone-iodine with ethanol three times, ending with povidone-iodine.
- Transfer the mouse to the previously prepared surgical work surface, with heating pad and sterile drape in place.
- Create a linear incision (3-5 mm) through the dorsal skin approximately 5 mm caudal to the bony prominence of the shoulder blade using iris scissors and standard pattern forceps (e.g. FST, 11271-30) by tenting the tissue and cutting parallel to the spine. The target incision site may be identified by transdermal visualization of the dorsal fat pad if skin pigmentation permits; cut at the caudal border of the fat pad. Expand the incision as needed, and extend through secondary skin layers using spring scissors.
- Anatomical note – The spinotrapezius muscle lies dorsally and extends from T4 to L3 along either side of the spine. The anterior border extends laterally to approximately the shoulder blade. The muscle tapers in the caudal direction so that the most posterior border lies medial to the anterior border, and may lay flush with the spine. Two fat pads are also important to note, laying directly dorsal and ventral to the spinotrapezius muscle towards the cranial half.
- Inflammation due to the incision has been shown to have no effect on the vascular remodeling response, as sham ligation surgery showed no change in remodeling of the spinotrapezius vasculature. Two spatial factors contribute to this outcome. First the incision is removed by 5 mm in the cranial direction from the region of interest where the vascular response in the muscle is typically examined. Additionally, the incision site on the dorsal skin is separated from the vessel ligation site by a ventral fat pad that lies on top of the cranial third of the spinotrapezius muscle.
- Irrigate the incision with warmed Ringer’s solution to prevent muscle desiccation. Application of vasodilators such as papaverine will assist in visualizing the target artery for ligation.
- Under a dissecting microscope, use forceps to blunt dissect and separate (but not remove) the dorsal white-fat pad from the underlying muscular tissue in order to visualize the vasculature as it enters and leaves the spinotrapezius on its lateral edge.
- Locate the target artery, which along with one or two paired veins, passes through the fat pad that is ventral to the spinotrapezius on its way in to the lateral edge of the muscle. This artery feeds a substantial portion of the caudal half of the muscle, which can be confirmed by reflecting and replacing the muscle to observe the path of the artery within the muscle. The target location for ligation is the segment of the artery exiting the ventral fat pad and entering the muscle, and is quite accessible in this region. The artery-vein pair may need to be gently separated from the ventral fat pad in this region. Note that the spinotrapezius is fed by multiple artery-vein pairs, including vessels that transverse either the dorsal and ventral fat pads. Take care not to disrupt these vessels.
- Multiple indicators may be used to distinguish artery from vein. One of the best methods is to gently obstruct blood flow with the microprobe and move upstream away from the muscle; flow will not resume in the artery until the obstruction is released. Color can also be used, as vein walls contain a whitish sheath of connective tissue that arteries lack. Further, one may consider the diameter before application of vasodilator as veins are typically larger in size.
- The artery is typically in close proximity to its paired vein; use blunt dissection with a microprobe and #5 forceps to isolate an approximately 5 mm segment, by passing the microprobe behind the artery, and using the tip to make a wider gap in the natural cleft between the artery and vein. This is the gap through which the suture will be threaded.
- Using 10-0 single-thread suture and needle holder place a ligation around the feed artery (~70 μm diameter) towards the proximal end of the dissected segment using a surgeon’s knot. Place an additional ligation towards the distal end of the dissected artery segment, with sufficient separation between ligatures to allow for transection of the artery.
- The small scale associated with the spinotrapezius feed artery leads to increased surgical difficulty, compared to ligation of a larger vessel such as the femoral artery. Inexperienced surgeons particularly will have high rates of avulsion, inadvertent hemorrhaging, and other surgical complications. Of particular concern is the difficulty of identifying and cutting the colorless artery after flow has been impeded just before cutting. For these reasons, placing 2 ligatures is recommended for those unaccustomed to the procedure, as the surgeon has a visual guide in locating where to transect the artery as well as a confirmation of the cut with the readily observable separation of the 2 ligatures. However, a more experienced surgeon may find a single ligature sufficient, as long as they are able to clearly identify and cut the artery downstream of the ligature. While this technique reduces the risk of unintentionally nicking a nearby vessel with the sharp ligation suture, it is not without drawbacks, including increased difficulty in confirming the artery was transected.
- Verify ligation by observing diminution of blood flow (obstructed RBC column) downstream of the ligation site (i.e. towards the muscle).
- Using dissecting scissors transect the ligated artery, between the two ligatures. If only one ligature was placed (11a), cut with extreme care in the downstream direction.
- Drug loaded plain-film (1 mm) may be placed downstream (caudally) as part of experimental procedure.
- Reposition the displaced fascia and adipose tissue to their original orientation.
- Close the skin incision using 8-0 non-resorbable sutures. Then place the mouse in a prepared heated recovery cage under observation and administer an appropriate analgesic such as bupivacaine, 0.02-0.05 ml 0.25% local infiltration.
2. Spinotrapezius Intravital Microscopy, in situ
- Anesthetize the mouse in an induction chamber with 3-4% isoflurane vaporized in 100% oxygen at a flow rate of 3-4 L·min-1.
- The use of inhalant anesthesia is preferable for functional measurements because there is less cardiovascular depression than with injectable anesthetics. If inhalant anesthesia in not available, long-acting injectable anesthetics, such as sodium pentobarbital are preferred to avoid repeat injections once the animal has been positioned.
- Once anesthetized, continually deliver isoflurane through an anesthesia mask (aka nose cone) at maintenance concentrations (generally ~ 2%) and flow rate of 0.5-1 L·min-1.
- Mice predominately ventilate through their nasal passages, so it is only necessary to cover their nose with the anesthesia mask diaphragm.
- If necessary, remove hair from the back using trimming clippers and depilatory cream.
- Transport the animal to the heat pad.
- It is important to maintain mice in a euthermic state to prevent cold-induced vasoconstriction; this can be accomplished through lamps, water circulating heat pads, microwavable heats pads, etc.
- Insert the rectal temperature probe and set thermo-controller to 35 °C.
- Make a skin incision at the caudal end of the spinotrapezius using iris scissors and standard pattern forceps.
- Extend the incision cranially to the fat pad, creating a horse shoe incision, and cover the skin flap with plastic wrap to prevent desiccation.
- Blunt dissect the subcutaneous connective tissue with forceps and spring scissors to maximize visibility.
- Place stimulating electrodes as close together as possible, to minimize the size of the current field, and position them at the caudal end of the exposed muscle just lateral to the spine.
- Perform a test stimulation to confirm electrode placement using an electrode data acquisition system, electrode stimulus isolator, and computer controller, with square waves of a 200 μsec duration, 2 mA amplitude, delivered at 1 Hz.
- Place plastic wrap over the exposed muscle to prevent desiccation.
- Begin timing a 30 min period during which vessel diameters will equilibrate.
- Position the intravital microscope over the spinotrapezius muscle to view the vascular architecture.
- If using an immersion lens, place a drop of PBS between the objective and the plastic wrap.
- Starting above the main arteriole (the largest vessel visible), manipulate the stage in the X-Y plane to locate the vessel of interest.
- Visualizing small arterioles with reflected-light microscopy requires greater contrast than provided by the red-cell column. We primarily use a side-stream dark-field imaging microscope to enhance microvascular contrast, but contrast can also be improved with a reflected-light fluorescent microscope after the intravenous injection of a high-molecular weight fluorescent dextran.
- After the 30-min equilibration, capture an image/video of the vessel of interest.
- Stimulate the muscle with square waves of 200 μsec duration, 2 mA amplitude, delivered at 8 Hz, for 90 sec, as described above.
- Capture another image/video immediately post stimulation and continue to capture every minute until vessel has returned to the resting diameter, ~10 min.
- Repeat steps 6-18 using the contralateral muscle.
- Data analysis can be performed in real time with video calipers or off-line with image analysis software.
- Euthanize the mouse following IACUC protocol.
3. Spinotrapezius Tissue Harvest and Fixation
- Anesthetize mice by intraperitoneal injection; formulation as in Spinotrapezius Feed Artery Ligation step 1.
- Make an incision several millimeters cranial to the bony prominence of the mouse’s shoulder blade using iris scissors and standard pattern forceps. Expand the incision laterally and then caudally on both sides.
- Release the skin from dorsal torso by gently reflecting the skin and cutting the superficial fascia.
- Use blunt dissection to remove dorsal adipose tissue using standard forceps, and then reflect the muscle and similarly dissect ventral adipose tissue.
- Remove fascia overlying the muscle using forceps and spring scissors. This step can be difficult and time consuming, but is important to improve immunofluorescent staining and imaging. Keeping the tissue hydrated can ease removal of the fascia.
- Locate the lateral border of the muscle, and use blunt dissection to separate the spinotrapezius muscle from the fat pad that lies ventral to it.
- Continue the blunt dissection in a cranial to caudal direction. Note that several blood vessels enter and leave this ventral surface of the muscle; if perfusion is required, it should be performed before transecting these vessels. Note also that in the caudal half of the muscle, its lateral border inserts into the muscle lying ventral to it. Some cutting may be required along this border to completely define and free the edge.
- Excise the spinotrapezius muscle. To do this:
- Free the lateral edge of the muscle as described above.
- Cut transversely across the muscle’s most cranial extent.
- Cut along the medial border (along the spine), in the sagittal plane.
- Repeat procedure steps 4-9 on the contralateral muscle, and then euthanize the mouse by an IACUC approved method.
- Fix the tissues on gelatin-coated glass slides by immersion in chilled methanol (4 °C) or in a 4% paraformaldehyde/deionized water solution at RT for 20 min.
- Alternatively, perfusion fixation may be employed. Prior to step 2, perform a thoracotomy, make an incision in the right atrium, and perfuse vasculature with 5 ml of 1×Tris-buffered saline with 0.1 mM CaCl2 and 2% heparin via the left ventricle, followed by 5 ml of 4% paraformaldehyde for vessel fixation, and finally 5 ml of 1×Tris-buffered saline with 0.1 mM CaCl2 7.
- Immediately following fixation, wash specimen four times for 20 min each using 0.01 M phosphate-buffered saline (PBS) with 0.1% saponin.
Representative Results
A surgical view of the main artery feeding the spinotrapezius muscle that undergoes ligation is shown in Figure 1, with labels indicating areas of interest. An example confocal image of the harvested and immunostained region of muscle located directly downstream from the ligated artery one week after ligation is shown in Figure 2A. Enlarged collateral arterioles containing smooth muscle alpha-actin expressing smooth muscle cells (red) display characteristic tortuosity following ligation. Vessel tortuosity, reported as the ratio between vessel path length and cord distance (ie, the line extending over the same vessel path endpoints), in the ligated muscle (right) is enhanced relative to unligated contralateral muscle (Figure 2B; n=8). Figure 3 shows the results of a pilot study in which terminal arteriole functional vasodilation was measured using intravital microscopy. As expected, arteriolar diameter significantly increases following electrical stimulation-induced muscle contraction.
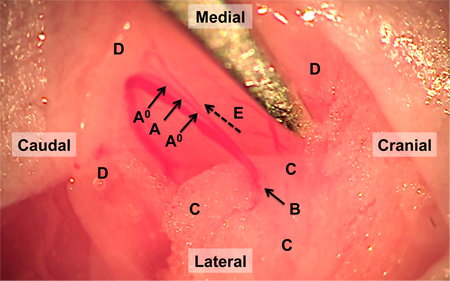
Figure 1. Surgical field of reflected spinotrapezius muscle and feed artery. The feed artery is ligated at the two locations indicated by A0. Between these ligatures and after obstruction of bloodflow, the artery is transected at the site indicated by A. The targeted segment of the artery transitions from the ventral fat pad, outlined by C, into the spinotrapezius muscle, outlined by D, at the site indicated with arrow B. Flow direction is indicated by dashed arrow E.
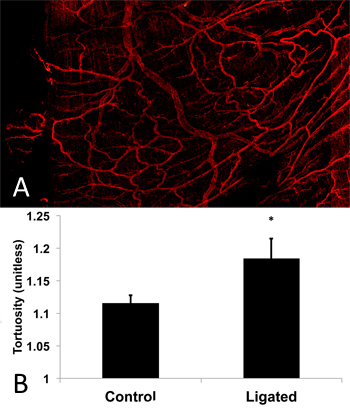
Figure 2. Confocal image of spinotrapezius muscle microvascular network for pilot study of tortuosity. (A) Harvested and whole-mounted muscle was immunostained for smooth muscle alpha-actin and imaged using confocal microscopy. The field of view shows the region of muscle located directly downstream of the ligated arteriole 1 week post ligation. Tortuous vessels are evident in the muscle as a result of the ligation. (B) Pilot study results show significantly increased (p=0.035; 1 tailed Student’s t-test) tortuosity exhibited by microvessels in the ligated muscle as measured by vessel path length to cord distance ratio for a trial of C57BL/6 mice (n=8) compared to contralateral control.
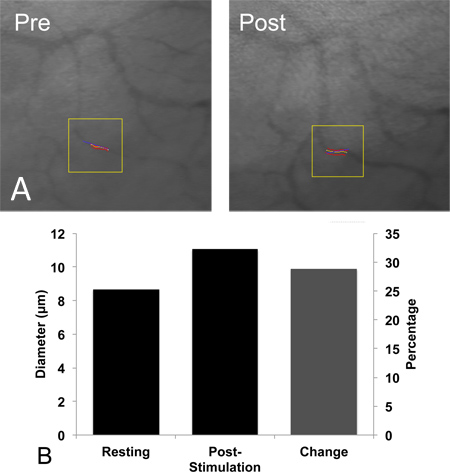
Figure 3. Intravital microscopy imaging of functional vasodilation in spinotrapezius arterioles, in vivo. (A) Representative photomicrographs of spinotrapezius terminal arterioles at rest (left) and immediately following the cessation of 8 Hz muscle contraction (right). (B) Terminal arteriole diameter at rest (8.5 ± 0.5 μm, left) and immediately following (11 ± 1 μm, middle) 90 sec of muscle contraction, which significantly increased arteriolar diameter (p=0.032; 1-tailed paired t-test) in Balb/C mice (n=6). Percent change indicated (right).
Discussion
The murine spinotrapezius ligation model presented here is an effective small animal model to study the functional and structural adaptations that result from an arterial occlusion. This model is complementary to the widely-used hindlimb ischemia model4, in that it provides a whole-muscle view of intact microvascular networks with high spatial resolution. Moreover, because this muscle is located just beneath the dorsal skin, it is accessible to serial imaging with intravital microscopy, and to local drug delivery via superfusion or thin film implantation2. These features make it an appealing small animal model in which to study the effects of novel therapeutic targets on vascular remodeling and functional vasodilation following arterial occlusion.
Note that caution should be used when comparing data acquired in the spinotrapezius ligation model to data acquired in the hindlimb ligation model due to several key differences. First, the spinotrapezius muscles are stabilizing muscles, and they differ from the leg muscles in terms of function and fiber type distribution. Our group has previously demonstrated that the arteriolar ligation in the spinotrapezius muscle creates less or negligible hypoxia in strains with well-developed collateral arteriolar networks, as compared to the hypoxia observed in the hindpaw following the femoral artery ligation in the hindlimb model1,8-10. We have also shown that the spinotrapezius ligation model produces a different remodeling response in Balb/c mice as compared to C57bl/6 mice, with Balb/c mice networks remodeling by capillary collateralization and C57bl/6 remodeling by enlargement of existing arteriolar connections3. There is an interesting parallel between these observations and those made in hindlimb ligation models, wherein Balb/c mice experience a prolonged perfusion recovery following ligation compared to C57bl/6 mice9,11,12. In other models of tissue ischemia, mouse age13 gender14 and the presence of disease15 also impact the tissues’ responses to arteriolar occlusion, although we have not yet tested these predictors in the spinotrapezius ligation model.
Second, the size of the artery that is ligated in the spinotrapezius model is substantially smaller than the femoral artery, and therefore, the ligation impacts a smaller tissue volume that is further downstream, i.e. at a lower level of the circulatory tree, in the spinotrapezius compared to the hindlimb3. Hence, the anatomical difference in the location of the ligation in these two models should be considered when comparing the respective physiological responses (e.g. vascular remodeling) to this intervention.
Third, the thinness of the spinotrapezius may permit oxygen to reach the unperfused portion of the tissue from neighboring tissue, compared to the combined ischemia and hypoxia in the hindlimb model as noted above3. Fourth, the recovery of flow to the portion of the spinotrapezius downstream of the ligation is faster than in the hindlimb ligation. The spinotrapezius ligation model is a unique chronic model and should not be confused with other models of transient arterial occlusion or models of ischemia reperfusion injury. We consider it possible to adapt this model to accommodate these types of conditions, but this is outside of the scope of work presented herein. Lastly, we caution against directly relating pre-clinical observations in this model to clinical manifestations of ischemic disease in humans, especially when utilizing young and otherwise healthy mice and due to the inherent physiological limits such as higher heart rate5. Nonetheless, we regard this new model as a valuable tool for uncovering basic mechanisms of tissue responses to ligation and identifying potential therapeutic targets in an in vivo setting.
In addition to the surgical notes provided in the spinotrapezius feed artery ligation procedure (anatomical note (6a), distinguishing the artery from vein (9a), and use of a single ligature (11a)), a few other aspects of the procedure benefit from discussion.
First, the initial incision made over the dorsal fat pad border (or several mm caudal to the shoulder blade, if transdermal visualization is not possible) is ideally made as small as the surgeon is comfortable with, and expanded during the procedure if needed. This practice minimizes the suturing required to close the incision, which is convenient for the surgeon, reduces procedure time, and is also less irritating to the animal during recovery. Alternatively, a larger skin incision may be appropriate according to surgeon preference, since the muscle can be easier to locate with an expanded field. In this case, the incision may benefit from a horse-shoe shape with a medial open face to allow folding towards the spine. While making the skin incision and extending through the secondary skin layers, if a surface vessel is damaged and causes hemorrhaging, apply light pressure with sterile gauze and provide time for hemostasis.
Also of note, it is important to handle the tissue with the minimum required force, and wherever possible hold with forceps closer to the muscle’s lateral border and away from the intended ischemic zone. This practice helps to avoid crush injury, which can lead to additional inflammatory-mediated tissue responses that may confound the vascular remodeling response evoked by the arterial ligation.
In conclusion, we have demonstrated the use of the murine spinotrapezius muscle as a surgical model for examining vascular and tissue responses to arteriolar ligation. This model is suitable for intravital assessment of vascular changes (for example, functional vasodilation), and for ex vivo assessment of vascular changes (for example, immunofluorescent imaging and quantitation of vascular networks). Both pre-clinical and clinical studies have demonstrated that an individual’s response to arteriolar obstruction (e.g. via ligation in mice or atherosclerotic plaques in humans) is dependent on their age the presence or absence of disease (e.g. diabetes mellitus) the diameter of the obstructed artery, the genetic makeup of the individual, and the metabolic demand of the tissue 11-15. Murine models, such as the one presented here, avail the researcher of strain-specific anatomical and genetic differences and disease-specific phenotypes, which facilitates investigation into these complex relationships.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We acknowledge Kevin Macleod for use of his creative commons licensed music in the associated video, including (in order of appearance) his tracks “Airport Lounge,” “Wallpaper,” and “Evening Melodrama.” We would also like to acknowledge Ndubisi Okeke and Frederick Torstrick for their assistance with the surgery video.
Materials
| Name | Company | Catalog Number | Comments |
| Iris scissors | FST | 14090-09 | Type: Tool |
| Size 7 forceps | FST | 11271-30 | Type: Tool |
| Size 5 forceps | FST | 11251-20 | Type: Tool |
| Spring scissors | Roboz | RS5671 | Type: Tool |
| Microprobe | FST | 10140-03 | Type: Tool May be substituted with straight probe |
| Needle holder | FST | 12500-12 | Type: Tool |
| Induction chamber | JD Medical Dist. Co., Inc. | IC-1086 | Type: Equipment |
| Eye Gel | Dechra | NDC 17033-211-38 | Type: Reagent |
| Heat pad | FST | 21060-01 | Type: Equipment |
| Rectal temperature probe | FST | 21060-01 | Type: Equipment |
| Stimulating electrodes | FHC | UEWSGCSE0N1M | Type: Equipment |
| Artisan’s Polymer Clay | Polyform | N/A | Type: Equipment |
| PowerLab data acquisition system | ADInstruments | ML 845 | Type: Equipment |
| Stimulus isolator | ADInstruments | FE 180 | Type: Equipment |
| LabChart | ADInstruments | ML S060/7 | Type: Software |
| Reflected-light fluorescent microscope | Olympus | BFXM | Type: Equipment |
| High MW fluorescent dextran | Sigma | FD250S-100MG | Type: Reagent |
| Video calipers | Colorado Video | 308 | Type: Equipment |
| Automated Vascular Analysis (AVA) | Microvision Medical | Type: Software | |
| Anti-αSMA Conjugated Fluorophore | Sigma | 1A4-Cy3 | Type: Reagent Clonal, 1:100 |
| Fluorescent Microscope | Olympus | BFXM | Type: Equipment |
| High-molecular weight fluorescent dextran | Sigma | FD250S-100MG | Type: Reagent |
Riferimenti
- Bailey, A. M., O’Neill, T. J., Morris, C. E., Peirce, S. M. Arteriolar Remodeling Following Ischemic Injury Extends from Capillary to Large Arteriole in the Microcirculation. Microcirculation. 15, 389-404 (2010).
- Bruce, A. C., Peirce, S. M. Exogenous Thrombin Delivery Promotes Collateral Capillary Arterialization and Tissue Reperfusion in the Murine Spinotrapezius Muscle Ischemia Model. Microcirculation. 19, 143-154 (2012).
- Mac Gabhann, F., Peirce, S. M. Collateral capillary arterialization following arteriolar ligation in murine skeletal muscle. Microcirculation. 17, 333-347 (2010).
- Niiyama, H., Huang, N. F., Rollins, M. D., Cooke, J. P. Murine Model of Hindlimb Ischemia. J. Vis. Exp. (23), e1035 (2009).
- Madeddu, P., et al. Murine models of myocardial and limb ischemia: diagnostic end-points and relevance to clinical problems. Vascul. Pharmacol. 45, 281-301 (2006).
- Cardinal, T. R., Kurjiaka, D. T., Hoying, J. B. Chronic hindlimb ischemia impairs functional vasodilation and vascular reactivity in mouse feed arteries. Front. Physio. 2, 91 (2011).
- Sefcik, L. S., et al. Selective Activation of Sphingosine 1-Phosphate Receptors 1 and 3 Promotes Local Microvascular Network Growth. Tissue Eng. Part A. 17, 617-629 (2011).
- Deindl, E., et al. Role of Ischemia and of Hypoxia-Inducible Genes in Arteriogenesis After Femoral Artery Occlusion in the Rabbit. Circulation Research. 89, 779-786 (2001).
- Chalothorn, D., Zhang, H., Smith, J. E., Edwards, J. C., Faber, J. E. Chloride Intracellular Channel-4 Is a Determinant of Native Collateral Formation in Skeletal Muscle and Brain. Circulation Research. 105, 89-98 (2009).
- Scholz, D., et al. Contribution of Arteriogenesis and Angiogenesis to Postocclusive Hindlimb Perfusion in Mice. Journal of Molecular and Cellular Cardiology. 34, 775-787 (2002).
- McClung, J. M., et al. Skeletal muscle-specific genetic determinants contribute to the differential strain-dependent effects of hindlimb ischemia in mice. Am. J. Pathol. 180, 2156-2169 (2012).
- Dokun, A. O., et al. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical hindlimb ischemia. Circulation. 117, 1207-1215 (2008).
- Faber, J. E., et al. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler. Thromb. Vasc. Biol. 31, 1748-1756 (2011).
- Peng, X., et al. Gender differences affect blood flow recovery in a mouse model of hindlimb ischemia. Am. J. Physiol. Heart Circ. Physiol. 300, 2027-2034 (2011).
- Li, Y., Guan, H., Hazarika, S., Liu, C., Annex, B. H. Impaired angiogenesis following hind-limb ischemia in diabetes mellitus mice. Chin. Med. Sci. J. 22, 232-237 (2007).

