Visualization and Genetic Manipulation of Dendrites and Spines in the Mouse Cerebral Cortex and Hippocampus using In utero Electroporation
Summary
This article describes in detail a protocol to electroporate in utero the cerebral cortex and the hippocampus at E14.5 in mice. We also show that this is a valuable method to study dendrites and spines in these two cerebral regions.
Abstract
In utero electroporation (IUE) has become a powerful technique to study the development of different regions of the embryonic nervous system 1-5. To date this tool has been widely used to study the regulation of cellular proliferation, differentiation and neuronal migration especially in the developing cerebral cortex 6-8. Here we detail our protocol to electroporate in utero the cerebral cortex and the hippocampus and provide evidence that this approach can be used to study dendrites and spines in these two cerebral regions.
Visualization and manipulation of neurons in primary cultures have contributed to a better understanding of the processes involved in dendrite, spine and synapse development. However neurons growing in vitro are not exposed to all the physiological cues that can affect dendrite and/or spine formation and maintenance during normal development. Our knowledge of dendrite and spine structures in vivo in wild-type or mutant mice comes mostly from observations using the Golgi-Cox method 9. However, Golgi staining is considered to be unpredictable. Indeed, groups of nerve cells and fiber tracts are labeled randomly, with particular areas often appearing completely stained while adjacent areas are devoid of staining. Recent studies have shown that IUE of fluorescent constructs represents an attractive alternative method to study dendrites, spines as well as synapses in mutant / wild-type mice 10-11 (Figure 1A). Moreover in comparison to the generation of mouse knockouts, IUE represents a rapid approach to perform gain and loss of function studies in specific population of cells during a specific time window. In addition, IUE has been successfully used with inducible gene expression or inducible RNAi approaches to refine the temporal control over the expression of a gene or shRNA 12. These advantages of IUE have thus opened new dimensions to study the effect of gene expression/suppression on dendrites and spines not only in specific cerebral structures (Figure 1B) but also at a specific time point of development (Figure 1C).
Finally, IUE provides a useful tool to identify functional interactions between genes involved in dendrite, spine and/or synapse development. Indeed, in contrast to other gene transfer methods such as virus, it is straightforward to combine multiple RNAi or transgenes in the same population of cells.
In summary, IUE is a powerful method that has already contributed to the characterization of molecular mechanisms underlying brain function and disease and it should also be useful in the study of dendrites and spines.
Protocol
In the United Kingdom, mice are housed, bred, and treated according to the guidelines approved by the Home Office under the Animal (Scientific Procedures) Act 1986.
1. Preparation: DNA Solution and Needles
- Purify plasmid DNA with an Endotoxin free Maxi-prep kit. Prepare plasmid DNA solution for injection to desired concentrations in water and add Fast Green (final concentration of 0.05%) to visualize injections. The efficiency of electroporation is highly dependent on the DNA concentration. A concentration of 1 μg/μl is generally used. This concentration is sufficient to visualize electroporated neurons without affecting their development. However, according to the promoter used in the plasmid vector (low expression level with cytomegalovirus promoter/enhancer, strong expression level with the cytomegalovirus immediate early enhancer and chicken β-actin promoter fusion (CAG) promoter) as well as the size and the stability of the protein expressed, this concentration can be adjusted (0.25 μg/μl to 5 μg/μl).
- Pull glass needles using a micropipette puller.
2. Preparation of the Surgery
- Autoclave surgical instruments and phosphate buffer saline (PBS).
- Prepare analgesic solution in PBS (Buprenorphine, Vetergesic, final concentration of 30 μg/ml). Weigh the pregnant mouse and inject subcutaneously 0.1 mg/kg of Vetergesic at least 30 minutes before the surgery. During this time, prepare the surgical area. Turn on the heating pads and the recovery chamber. Place sterile PBS in warm water bath and all sterile instruments and materials on sterile drapes. Place the platinum electrodes into a PBS filled beaker and connect to the electroporator. Fill the needle with the DNA solution using a microloader tip, connect the needle to the capillary holder and pinch off the tip of the needle with a forceps.
3. Surgery Before DNA Injection and Electroporation
- Anaesthetize a pregnant mouse (E14.5-E15.5) with isoflurane in oxygen carrier (oxygen 2 l/min) using an anaesthetic induction chamber. Wait until the animal loses righting reflex.
- Transfer the animal to a “pre-surgery” mask. Place a drop of eye gel on each eye to prevent corneal ulceration of the eyes while the mother is under general anesthesia. Use an electric razor to shave the hair of the abdomen. Clean the shaved area once with clorhexidine to collect flying hair.
- Transfer the animal to a second mask in the surgical area. Place the mouse with its back on the heating pad. Start the surgery when the pedal reflex has been lost.
- Put on mask and sterile gloves. Cover the animal with a sterile drape (with a small hole over the abdomen) to prevent the tissues and instruments from being contaminated by the areas of skin that have not been shaved and disinfected. Clean the shaved area at least 3 times with clorhexidine. Use a different sterile cotton swab each time. Use a scalpel to make a vertical incision along the midline (~1 inch long) through the skin. Using scissors, make a similar incision of the muscle of the abdomen along the linea alba (white line composed mostly of collagen connective tissue).
- Choose the most accessible embryos and place the ring forceps between two embryos and carefully pull the embryonic chain out of the abdominal cavity. From this point on, keep the embryos hydrated with sterile pre-warmed PBS.
No microscope is required for visualization.
4. Injection of DNA and Electroporation
- Start with one of the most lateral embryos, making it easier to keep track of which embryos were electroporated. Do not pull too much on the embryos as this will increase risk of hemorrhage. Manipulate the position of the embryo inside the amniotic sac using the ring forceps and stabilize the head of the embryos between the rings. Squeeze gently to push up the embryo closer to the uterine wall.
- With the other hand, take the capillary holder and insert the needle carefully into the middle of the hemisphere to target the lateral ventricle. Press the pedal to inject approximately 1 μl of DNA solution mixed with Fast Green (less than 1 μl to study dendrites). You should observe the green dye filling the lateral ventricle. Critical steps: – The sharpness of the needle is critical to pierce properly the uterine wall. It is important to minimize the movement of the needle at the surface of the uterine wall and after insertion into the ventricle because an enlargement of the hole will result in the leakage of amniotic fluid and embryo death. Avoid piercing blood vessels in the uterine wall, as this will result in bleeding and embryo death.
– It is important not to inject a too large volume of DNA into the lateral ventricle because it will induce hydrocephalus (do not exceed 2 μl at E14.5). The volume of DNA is adjusted according to the purpose of the experiment. If 1μl is generally used for most experiments, a smaller volume of DNA (approximately 0.5 μl) is required for dendrite and spine analysis. Indeed a few isolated cells need to be targeted in order to visualize and measure the dendritic arbor of electroporated neurons. - Place the electrodes on the sides of the embryo head with the positive (+) paddle on the same side as the injected ventricle for cortex electroporation or on the opposite side of the injected ventricle for hippocampus electroporation (Figure 2). Then apply five 30V electrical pulses (50 msec duration) at 1 sec intervals. Critical step: Avoid applying current across the placenta as this will result in embryo death.
All the embryos in a pregnant mouse can be electroporated, usually with the same DNA construct to avoid any confusion. However, a long surgery decreases the survival rate of embryos. The abdominal cavity should not be opened longer than 30 min.
5. Surgery Post-electroporation
- After electroporating the embryos, add PBS into the abdominal cavity and use the ring forceps to replace the uterine horn in its original location. Suture the abdomen wall and skin with Vicryl absorbable sutures.
- Place the animal in a recovery chamber until it wakes up (usually 5-10 min) and then transfer in a cage placed on a heating pad.
6. Post-surgery
Check the behavior of the mice to assess pain, suffering or distress and weigh the animals 24 h and 48 h after the surgery. If needed, analgesics can be administered to minimize pain and discomfort.
7. Tissue Processing
Collect the electroporated embryos or pups at the embryonic or postnatal stages required for the experiment.
– For analysis at embryonic stages (for example to study cell proliferation or migration):
Euthanize mother via cervical dislocation and collect the embryos. After decapitation, select the brains that have been properly electroporated, as indicated by the amount and location of the fluorescent signal, visualized across the skull using a fluorescent binocular. Dissect the brain out of the skull and fix overnight in 4% PFA and then place in 20% sucrose / PBS overnight. Embed in OCT compound, freeze at -80 °C and section using a cryostat.
– For analysis at postnatal stages (for example to study dendrites and spines):
Anesthetize pups or adult mice with intraperitoneal injection of pentobarbitone (40-60 mg/kg) and perform transcardial perfusion with PBS, followed by 4% PFA in PBS. Dissect the brains out of the skull and post-fix in 4% PFA overnight. After washings in PBS, section the brains using a vibratome (100 μm sections for dendrite analysis). Mount the sections in Aqua Poly/mount using 0.16-0.19 mm thick coverslips to image dendrites and spines.
8. Representative results
Figure 3 shows examples of electroporated cells in the cerebral cortex (Figures 3A, B), in the CA1 (Figures 3C, D) and in the dentate gyrus of the hippocampus (Figures 3E, F). Wild-type mice were electroporated at E14.5 with a GFP construct (pCA-b-EGFPm5 silencer 3) and brains were harvested at postnatal day (P) 14. By injecting a small volume of DNA solution (0.5 μl or less of a solution at 1 μg/μl), a few cells are labeled, which allows the visualization of the dendritic arborization of isolated GFP+ cells (Figure 3) as well as their spines at higher magnification (Figure 4).
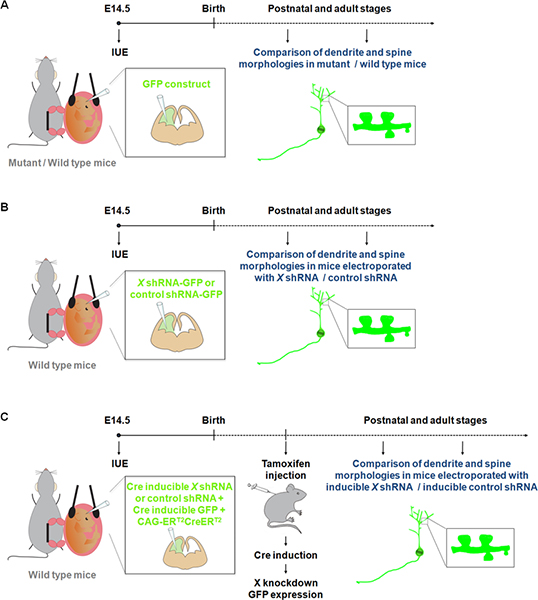
Figure 1. Schematic representation of electroporation protocols that can be used to study dendrites and spines. (A) Electroporation of a GFP construct to compare dendrites and spines in wild-type and mutant mice. (B) Electroporation of GFP-shRNA (GFP is expressed from the same construct) to compare dendrites and spines in mice electroporated with a shRNA construct specific for a gene of interest (X) or a control shRNA. (C) IUE can be used together with a Cre inducible system in order to restrict expression of the shRNA to the desirable time period. In this experiment, a vector expressing a form of the Cre recombinase which can be activated by 4-hydroxytamoxifen (CAG-ERT2CreERT2; 1 μg/μl) 10, is electroporated together with a vector expressing a specific shRNA in a Cre dependent manner (1 μg/μl, and with a recombination indicator (CALNL-GFP construct, GFP expression inducible by Cre; 1 μg/μl) 10. The efficiency of the knockdown can be improved by increasing the concentration of the shRNA as well as increasing CAG-ERT2CreERT2 concentration.
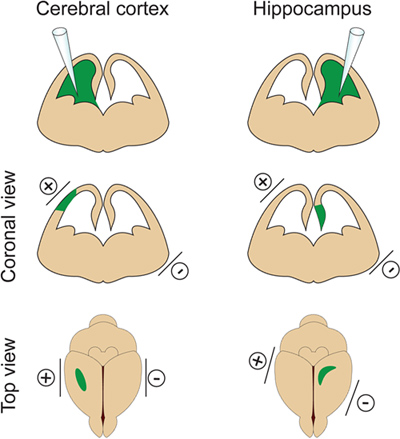
Figure 2. Spatial control of electroporation. This figure shows where to position the paddle electrodes according to the DNA injection site in order to target the cerebral cortex or the hippocampus.
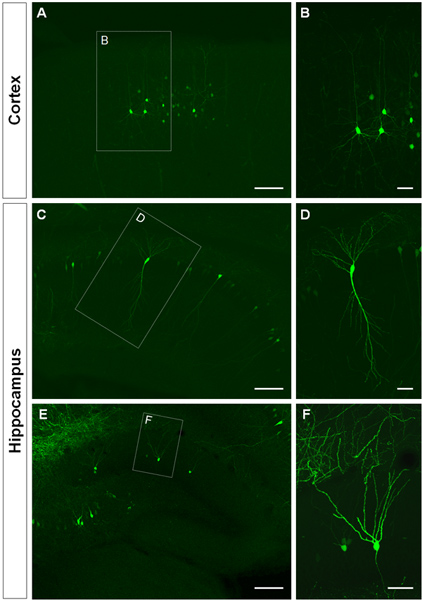
Figure 3. Visualization of the dendritic arbor of in utero electroporated cells in the cerebral cortex and hippocampus. (A, B) Coronal sections showing GFP+ pyramidal cells in the cerebral cortex, (C-D) pyramidal cells in CA1 of the hippocampus and (E, F), granule cells in the dentate gyrus at P14. A GFP construct (pCA-b-EGFPm5 silencer 3) was electroporated at E14.5. Higher magnification pictures (B, D, F) show that IUE is an efficient method to visualize dendrites. Scale bars represent 50 μm (B, D and F), 150 μm (A, C, E).
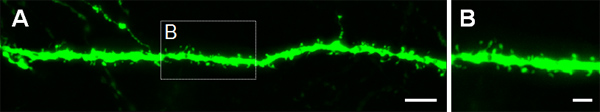
Figure 4. Visualization of dendritic spines in P14 neurons that were electroporated in utero at E14.5 with a GFP expressing construct. (A, B) High magnification images of spines from basal dendrites of hippocampal pyramidal neurons. Scale bars represent 5 μm (A) and 2 μm (B).
Discussion
IUE is a powerful tool to manipulate gene expression not only in space but also in time. We show here that this technique can be used to visualize and genetically manipulate dendrites and spines in the cerebral cortex and hippocampus of mice. Besides the advantages previously cited, it is worth noting that IUE, in contrast to Golgi method, can be combined with immunohistochemistry or in situ hybridization, which allows for example to phenotype the electroporated cells. It is also important to mention that this procedure does not induce evident brain malformations despite its relative invasiveness. In addition, at the cellular level, IUE does not modify the electrophysiological properties of the electroporated neurons13. While our demonstration focuses on the visualization of dendrite and spine morphologies, IUE of cortical or hippocampal neurons at E14.5 could also be used to study other developmental events such as axon formation and guidance. In addition, the same kind of protocol could be implemented at other stages of embryonic development to target different populations. For example, a developmentally very late cortical electroporation paradigm at E18.5 can be performed to drive expression in astrocytic progenitors 1. Similarly, while an electroporation of the hippocampus at E14.5 allows to target CA1-CA3 pyramidal neuron progenitors and dentate granule cell progenitor at the same time, a late hippocampal electroporation (E18.5 or early postnatal) would allow to target different dentate granule progenitors 14. In this case, the injected volume of DNA can be increased as well as the intensity of current.
Transgenes introduced by IUE appear to remain episomal and are therefore lost from cells following successive cell divisions. In postmitotic cells such as neurons, however, the episomal transgenes remain active for months after electroporation allowing long-term studies 13,15. In our study, we have observed bright GFP+ cells up to 7 weeks after birth (the latest time point we analyzed) indicating that embryonic targeting of cortical or hippocampal neuronal precursors using IUE results in persistent expression of the transgene from early developmental time points up to adulthood.
A current limitation of the technique is that it is difficult to exert a fine control over the total number of electroporated cells. However, by decreasing the injected volume of DNA solution, we have shown that it is possible to label a few cells and to visualize the dendritic arborization of isolated GFP+ cells as well as their spines. The dimension of the transfected area could also be adjusted by modifying the parameters of the electroporation such as intensity of current and number of pulses or the diameter of the electroporation paddles.
Altogether IUE is a method that is easy to implement, rapid and efficient to study dendrites and spines in vivo.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Dr. Kathleen Mathers, Dr. Jean-Philippe Mocho and Dr. Yolanda Saavedra Torres for their help to perform in utero electroporation under aseptic procedures, and Hayley Wood for her help to prepare the drawings.
E.P. was supported by a long-term Federation of European Biochemical Societies (FEBS) fellowship and a Medical Research Council (MRC) career development fellowship, M.A.H. by a Wellcome Trust grant to Elizabeth Fisher and Victor Tybulewicz (080174/B/06/Z), H.W. by an EMBO long-term fellowship and R.A. by an MRC studentship. This work was supported by a project grant from the Wellcome Trust (086947/Z/08/Z) and by a Grant-in-Aid from the Medical Research Council (U117570528) to F.G.
Materials
| Name of the reagent | Company | Catalogue number | Comments | |||
| Preparation of needles and DNA solution for injection | ||||||
| Endofree Plasmid Maxi Kit | Qiagen | 12362 | ||||
| Fast Green | Sigma | F-7258 | ||||
| Borosilicate glass capillaries 1.0 mm O.D. x 0.58 mm I.D. |
Harvard Apparatus | 30-0016 | ||||
| Microloader tips | Eppendorf | 5242956003 | For Eppendorf pipettes 0.5 μl-10 μl / 2-20 μl | |||
| Material for surgery | ||||||
| Extra thin Iris scissors | Fine Science Tools | 14088-10 | ||||
| Curved Forceps | Fine Science Tools | 91197-00 | ||||
| Ring forceps | Fine Science Tools | 11103-09 | ||||
| Needle holder | Fine Science Tools | 12002-12 | ||||
| Graefe Forceps | Fine Science Tools | 11050-10 | ||||
| Vicryl absorbable suture | Ethicon Inc (Johnson & Johnson) | W9074 | ||||
| Sterile drapes 30cm x 45 cm | Buster | 141765 | ||||
| Sterile swabs | Shermond | HUBY-340 | ||||
| Cotton buds | Clean Cross Co., Ltd | 1860 | ||||
| Buprenorphine (Vetergesic) | Alstoe Animal Health | |||||
| Clorhexidine | Vetasept | XHG007 | ||||
| Eye gel Viscotears | Novartis | |||||
| Isoflurane | Abbott Laboratories | B506 | ||||
| Pentoject, Pentobarbitone Sodium 20% | Animalcare | |||||
| Electroporation | ||||||
| Electroporator | BTX | ECM830 | ||||
| Platinum Tweezertrode 5mm | BTX, Harvard Apparatus | 45-0489 | ||||
| Femtojet microinjector | Eppendorf | 5247000030 | ||||
| Foot Control for Femtojet Microinjector | Eppendorf | 5247623002 | ||||
| Capillary holder | Eppendorf | 5176190002 | ||||
| Tissue processing | ||||||
| Paraformaldehyde | Sigma | P6148 | ||||
| Sucrose | VWR (Prolabo) | 27480.294 | ||||
| Microscope slides | ThermoScientific (Menzel-Gläser) | J1800AMNZ | ||||
| Coverslips | Menzel-Gläser | 22 x 50 mm #1,5 | ||||
| Aqua Poly/mount | Polysciences, Inc | 18606 | ||||
Riferimenti
- Kolk, S. M., de Mooij-Malsen, A. J., Martens, G. J. Spatiotemporal Molecular Approach of in utero Electroporation to Functionally Decipher Endophenotypes in Neurodevelopmental Disorders. Front Mol. Neurosci. 4, 37 (2011).
- Saito, T., Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 240, 237-246 (2001).
- Shimogori, T., Ogawa, M. Gene application with in utero electroporation in mouse embryonic brain. Dev. Growth Differ. 50, 499-506 (2008).
- Tabata, H., Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscienze. 103, 865-872 (2001).
- Tabata, H., Nakajima, K. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev. Growth Differ. 50, 507-511 (2008).
- Castro, D. S. A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 25, 930-945 (2011).
- Pacary, E. Proneural transcription factors regulate different steps of cortical neuron migration through Rnd-mediated inhibition of RhoA signaling. Neuron. 69, 1069-1084 (2011).
- Marteau, L. Angiopoietin-2 regulates cortical neurogenesis in the developing telencephalon. Cereb Cortex. 21, 1695-1702 (2011).
- Ramon Moliner, E. . Comparative Methods in Neuroanatomy. , (1970).
- Banks, G. T. Behavioral and other phenotypes in a cytoplasmic Dynein light intermediate chain 1 mutant mouse. J. Neurosci. 31, 5483-5494 (2011).
- Elias, G. M., Elias, L. A., Apostolides, P. F., Kriegstein, A. R., Nicoll, R. A. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc. Natl. Acad. Sci. U.S.A. 105, 20953-20958 (2008).
- Matsuda, T., Cepko, C. L. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. U.S.A. 104, 1027-1032 (2007).
- Navarro-Quiroga, I., Chittajallu, R., Gallo, V., Haydar, T. F. L. o. n. g. -. t. e. r. m. selective gene expression in developing and adult hippocampal pyramidal neurons using focal in utero electroporation. J. Neurosci. 27, 5007-5011 (2007).
- Nakahira, E., Yuasa, S. Neuronal generation, migration, and differentiation in the mouse hippocampal primoridium as revealed by enhanced green fluorescent protein gene transfer by means of in utero electroporation. J. Comp. Neurol. 483, 329-340 (2005).
- Ramos, R. L., Bai, J., LoTurco, J. J. Heterotopia formation in rat but not mouse neocortex after RNA interference knockdown of DCX. Cereb Cortex. 16, 1323-1331 (2006).

