Mouse Model of Intraluminal MCAO: Cerebral Infarct Evaluation by Cresyl Violet Staining
Summary
The intraluminal middle cerebral occlusion model in mice is herein presented. The extent of cerebral infarct is evaluated by a neurologic score and cresyl violet staining, an alternative staining to TTC, offering the great advantage to test in parallel many interest markers.
Abstract
Stroke is the third cause of mortality and the leading cause of disability in the World. Ischemic stroke accounts for approximately 80% of all strokes. However, the thrombolytic tissue plasminogen activator (tPA) is the only treatment of acute ischemic stroke that exists. This led researchers to develop several ischemic stroke models in a variety of species. Two major types of rodent models have been developed: models of global cerebral ischemia or focal cerebral ischemia. To mimic ischemic stroke in patients, in whom approximately 80% thrombotic or embolic strokes occur in the territory of the middle cerebral artery (MCA), the intraluminal middle cerebral artery occlusion (MCAO) model is quite relevant for stroke studies. This model was first developed in rats by Koizumi et al. in 1986 1. Because of the ease of genetic manipulation in mice, these models have also been developed in this species 2-3.
Herein, we present the transient MCA occlusion procedure in C57/Bl6 mice. Previous studies have reported that physical properties of the occluder such as tip diameter, length, shape, and flexibility are critical for the reproducibility of the infarct volume 4. Herein, a commercial silicon coated monofilaments (Doccol Corporation) have been used. Another great advantage is that this monofilament reduces the risk to induce subarachnoid hemorrhages. Using the Zeiss stereo-microscope Stemi 2000, the silicon coated monofilament was introduced into the internal carotid artery (ICA) via a cut in the external carotid artery (ECA) until the monofilament occludes the base of the MCA. Blood flow was restored 1 hour later by removal of the monofilament to mimic the restoration of blood flow after lysis of a thromboembolic clot in humans. The extent of cerebral infarct may be evaluated first by a neurologic score and by the measurement of the infarct volume. Ischemic mice were thus analyzed for their neurologic score at different post-reperfusion times. To evaluate the infarct volume, staining with 2,3,5-triphenyltetrazolium chloride (TTC) was usually performed. Herein, we used cresyl violet staining since it offers the opportunity to test many critical markers by immunohistochemistry. In this video, we report the MCAO procedure; neurological scores and the evaluation of the infarct volume by cresyl violet staining.
Protocol
Transient Middle Cerebral Artery Occlusion (MCAO)
1. Surgery Procedure (Figure 1)
Transient middle cerebral artery occlusion (tMCAO) is performed on 2- to 3-month old male C57Bl/6 mice (22-28g). This protocol was approved by the IRCM bioethics committee animal care. Surgical tools were sterilized by autoclaving (121 °C at 15 psi for 60 min). Between each animal, they were sterilized using the hot bead sterilizer (15 sec). Surgery table and other equipment are sanitized using 70% ethanol.
Two hours before surgery, mice were analgesized with buprenorphine (0.03 mg/kg b.w. i.p.).
Figure 1A.
- Deeply anaesthetize mice with isoflurane 5% and then maintain the anesthesia at 2.5% isoflurane. Body temperature of the mice is maintained constant during surgery with a heating pad.
- Disinfect the fur and skin with 70% ethanol or Betadine. Since shaving produces hair fragment release, microabrasions and inflammation that may impact on stroke pathophysiology, we do not shave mouse fur.
- Make a midline neck incision and gently pull apart the soft tissues.
- Under a stereo microscope (Stemi 2000, Zeiss), a blunt dissection is performed to expose the trachea and retract the muscles to locate the carotid artery.
- The left common carotid artery (CCA) is carefully dissected from surrounding tissue and the CCA is temporarily occluded by a temporary suture (1) using 5-0 silk suture cut into 20 mm segments. Great care should be exercised not to harm the vagal nerve.
- Separate the bifurcation of the left internal common carotid artery (ICA) and external common carotid artery (ECA). A permanent suture (2) is placed around the ECA, as distally as possible, and another temporary suture slightly tight (3) is placed on the ECA distal to the bifurcation.
- Clip the left ICA (4) using a reverse-action tweezers to avoid bleeding. Great care should be exercised not to harm the vagal nerve.
- Cut a small hole into ECA (5) between permanent (2) and temporary (3) sutures.
Figure 1B.
- Introduce a 12 mm-long 6-0 silicon-coated (about 9-10mm is coated silicon) monofilament suture (Doccol Corporation) into the ECA (6), completely cut the ECA distal to the permanent suture and invert the occluder into the ICA. The suture is tightly tied around the monofilament to prevent bleeding and the reverse-action tweezers are removed.
- The occluder is introduced to occlude the origin of the MCA in the circle of Willis. Stop its insertion around 9-10 mm beyond the bifurcation of ECA and CCA. The occluder is blocked and cannot move anymore. Care must be exercised not to penetrate the pterygopalatine artery. The suture (3) on the ECA is tightly tied to fix the monofilament in position.
- Close the skin with an autoclip wound closing system.
- Inject 1 ml of saline solution subcutaneously and place mice under an infrared heating lamp during all the post-occlusion period (60 min).
2. Restoration of Middle Cerebral Artery Blood Flow
Before reanesthesia, the neuroscore can be checked to evaluate the success of the surgery.
- Anesthetize the mice as previously described and remove the autoclips.
- Slightly open the suture (3) onto the ECA to allow monofilament withdrawal and blood reperfusion.
- Permanently tie off the temporary suture on the ECA to prevent blood loss. The monofilament is kept for reuse.
- Remove the temporary suture (1) onto the CCA to allow blood recirculation.
- Close the wound. The mice should receive another 1 ml saline solution subcutaneously.
- Place the mice under the infrared heating lamp for 1 hr. After checking that the animal regains mobility, the mouse is returned to its cage and left under the heating pad and towels for 72 hr. Note that only half of the cage is placed on the heating pad to allow mice to choose their environment. Because post-surgical weight loss is generally observed, mashed food is placed in a Petri dish to encourage eating.
Twelve hours post-surgery, mice received another dose of buprenorphine (0.03 mg/kg b.w. i.p).
3. Sham Operation
For sham operations, all procedures are identical except that the occluder is not inserted.
4. Neuroscore
Neurological deficits allow the evaluation of the success of tMCAO just after reperfusion and later the estimation of the degree of severity of the injury. Neurological deficits are scored as previously described 5 and performed at 1, 24, 48 and 72 hr post-reperfusion. An expanded six-point scale is used:
- 0: normal.
- 1: mild circling behavior with or without inconsistent rotation when picked up by the tail, <50% attempts to rotate to the contralateral side.
- 2: mild consistent circling, >50% attempts to rotate to the contralateral side.
- 3: consistent strong and immediate circling, the mouse holds a rotation position for more than 1-2 sec, with its nose almost reaching its tail.
- 4: severe rotation progressing into barreling, loss of walking or righting reflex.
- 5: comatose or moribund.
5. Cresyl Violet Staining (Figure 2)
Following PBS solution perfusion, brains are quickly frozen in isopentane and stored at -80 °C. Mouse brains may be also perfused with 4% paraformaldehyde (PFA) depending on the planned immunohistochemistry studies. Cryostat-cuts of coronal brain sections (17 μm) are performed. One section out of every 30 is collected on the same slide to have a representative cerebral injury. For volume quantification, 2 slides are stained by cresyl violet staining and an image analysis system (Scion Corporation, Frederick, MD) was used to evaluate the lesion. The injury volume was calculated in arbitrary units (pixels), and expressed as a percentage of the contralateral non-lesioned area for each section. Other slides are kept at -80 °C for immunohistochemistry studies. Alternatively, PFA-fixed brain can be cut at 30-50 μm using vibratome and the infarct volume measured as recommended by Han et al. (2009)6.
Representative Results
The neuroscore evaluation confirms the success of tMCAO post-stroke and determines the efficiency of the tMCAO post-reperfusion. In our hands, all mice subjected to the intraluminal procedure presented at least a mild consistent curling (neuroscore 2) and the neurological deficits are generally stable up to 72 hr. Most mice exhibit a mild consistent curling (neuroscore 2). Mice showing absence of curling are excluded from the study. However, the neuroscore evaluation does not allow us to distinguish between mice with an injured hippocampus and those with an intact hippocampus.
No mortality was observed during the surgery day, suggesting that subarachnoid hemorrhages did not occur. When subarachnoid hemorrhage is identified in a mouse, this one is systematically excluded from analysis. The use of silicon-coated monofilament from Doccol Corporation, which is smoother than home-prepared monofilaments, increases the success of tMCAO and reduces subarachnoid hemorrhages. Mortality between 24 hr and 72 hr is 14% in this model, as generally reported for 60 min of occlusion. The mortality observed is probably due to a large infarct volume in this mouse strain. A strong lesion reproducibility has also been observed (standard deviation is 15%), which is very interesting to study neuroprotection molecules where their effect(s) could be hidden by the variability of the model.
To evaluate the extent of brain injury following tMCAO, we opted to use cresyl violet staining (Figure 2) rather than TTC in order to have a lot of materials to test relevant markers6. The extent of the lesion is relatively consistent. However, we noticed that in some mice, the hippocampus was injured (around 30% of mice). It is interesting to note that cresyl violet staining can be applied up to 1 week later. In the literature, the percentage of brain infarct varies from one study to the other. It depends on the choice of mouse strain, the anesthesia, the thickness of brain sections, the monofilament used, or the staining used 6, 7.
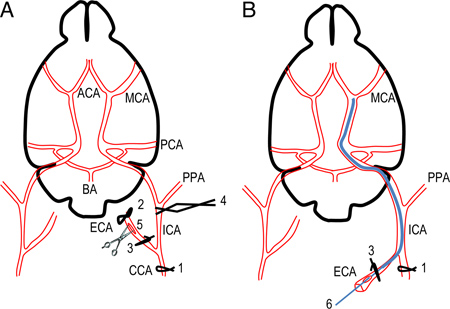
Figure 1. Scheme of the occlusion of the middle cerebral artery using silicon-coated intraluminal monofilament. A. Simplified scheme of mouse brain and cerebral arteries showing successive sutures and clip to prepare the introduction of silicon-coated monofilament. B. The position of monofilament through the circle of Willis is represented. The monofilament is introduced into ICA via ECA to occlude the base of the MCA. ACA, anterior cerebral artery; BA, basilar artery; CCA, common carotid artery; C. Willis, Circle of Willis; ECA, external carotid artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior communicating artery; PPA, pterygopalatine artery.
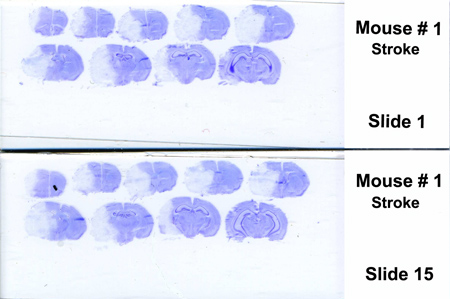
Figure 2. Representative coronal sections of mouse brain stained with cresyl violet after 72 hr post-reperfusion. Infarct area (mainly striatum, cortex and adjacent brain areas) appears in white (unstained by cresyl violet). The injury volume (white part, right hemisphere) was delimited and expressed as a percentage of the contralateral non-lesioned area (left hemisphere).
Discussion
Different stroke models have been developed to mimic stroke consequences in patients. The choice of the stroke model depends on the biological question. The intraluminal MCAO model mimics one of the most common types of ischemic stroke in patients and is less invasive and more consistent than the Tamura model 4,7. It is a really interesting model for neuroprotection, neurorepair and cell death analyses. The success of the intraluminal model depends on many factors such as animal sex, age and weight, temperature, anesthesia and the time of surgery, which have to be controlled. The physical proprieties of the occluder (tip diameter, length, shape and fexibility) are critical for the consistency of the MCAO 4. Herein, we use the 12 mm-long 6-0 monofilament coated with silicon on 9-10 mm from Doccol Corporation. The great advantage of this monofilament is to reduce subarachnoid hemorrhages and because its length covered all the ICA length, residual blood flow, which comes from the anterior and posterior communicating arteries of the Circle of Willis and PPA, is prevented and the variability in the infarct volume decreases (15% of variability in our hands) 8. The suture of CCA also decreases the variability of the infarct volume 9.
The extent of brain injury following tMCAO can be assessed by different ways. Neurological deficits can be measured as previously mentioned. However, it is difficult to have an efficient measure by this way. The extent of the infarct is commonly performed by TTC staining. More recently, magnetic resonance techniques are also used, a particularly interesting technology for neuroprotective treatments. To understand the underlying cellular mechanisms involved in stroke such as neurorepair, cell death or cell proliferation, the study of different markers by immunohistochemistry is critical and very informative. Thus, our protocol of brain section preparations has the great advantage of allowing these analyses, and minimizes the number of mice used. Moreover, Tureyen et al. 10 have reported that there is good correlation between cresyl violet staining and TTC.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study was supported by a CIHR TEAM grant # CTP 82946, a CIHR grant # MOP 102741, as well as a Canada chair # 216684 and a Strauss Foundation. E.R. was supported by a fellowship from the Canadian Heart & Stroke Foundation.
Materials
| Description | Company | Catalogue number |
| Stereo microscope | Zeiss | Stemi 2000 |
| Fiber-Light High Intensity Illuminator | Dolan-Jenner Industries Inc. | |
| Anesthesia system for isoflurane | ||
| Isoflurane | CDMV | 108737 |
| Heating blanket | Gaymar Medsearch | TP22G |
| Infrared heating lamp | ||
| Ultra fine tweezers, style 5 | Electron Microscopy Sciences | 78320-5TI |
| Vannas Scissors 3 mm Cutting Edge, Straight | Electron Microscopy Sciences | 72932-01 |
| Style LA-1 and MPF-1 All smooth blades | Electron Microscopy Sciences | 77926-5S |
| Negative action tweezers, style KOPINC 5 | Electron Microscopy Sciences | 72850-F |
| Dissecting scissors 5 1/2″ Straight | Cedarlane | 72960 |
| Autoclip starter set | Harvard apparatus | 34-0557 |
| BD Autoclip Wound Closing Syst., 9mm long,100/pk | Fisher | 01-804-5 |
| 5-0 suture | Harvard apparatus | 517763 |
| Suture material PDS II monofilament violet, 5-0, RB-1; Z303H | CDMV | 6167 |
| 12 mm-long 6-0 silicon-coated monofilament suture | Doccol Corporation | 60SPRePK5 |
| Seringes 1ml | Fisher | 14-823-2F |
| Needles 26G1/2 | Fisher | BD-305111 |
| C57BL/6 mice | IRCM |
Riferimenti
- Koizumi, J., Yoshida, Y., Nakazawa, T., Ooneda, G. Experimental studies of ischemic brain edema, I: a new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn. J. Stroke. 8, 1-8 (1986).
- Chan, P. H., Kamii, H., Yang, G., Gafni, J., Epstein, C. J., Carlson, E., Reola, L. B. r. a. i. n. infarction is not reduced in SOD-1 transgenic mice after a permanent focal cerebral ischemia. Neuroreport. 5, 293-296 (1993).
- Yang, G., Chan, P. H., Chen, J., Carlson, E., Chen, S. F., Weinstein, P., Epstein, C. J., Kamii, H. Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke. 25, 165-170 (1994).
- Liu, S., Zhen, G., Meloni, B. P., Campbell, K., Winn, H. R. Rodent stroke model guidelines for preclinical stroke trials (1st edition. J. Exp. Stroke Transl. Med. 2, 2-27 (2009).
- Jiang, S. X., Lertvorachon, J., Hou, S. T., Konishi, Y., Webster, J., Mealing, G., Brunette, E., Tauskela, J., Preston, E. Chlortetracycline and demeclocycline inhibit calpains and protect mouse neurons against glutamate toxicity and cerebral ischemia. J. Biol. Chem. 280, 33811-33888 (2005).
- Han, R. Q., Ouyang, Y. B., Xu, L., Agrawal, R., Patterson, A. J., Giffard, R. G. Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth. Analg. 108, 280-287 (2009).
- Rousselet, E., Marcinkiewicz, J., Kriz, J., Zhou, A., Hatten, M. E., Prat, A., Seidah, N. G. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J. Lipid Res. 52, 1383-1391 (2011).
- Tamura, A., Graham, D. I., McCulloch, J., Teasdale, G. M. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 1, 53-60 (1986).
- Chen, Y., Ito, A., Takai, K., Saito, N. Blocking pterygopalatine arterial blood flow decreases infarct volume variability in a mouse model of intraluminal suture middle cerebral artery occlusion. J. Neurosci. Methods. 174, 18-24 (2008).
- Tsuchiya, D., Hong, S., Kayama, T., Panter, S. S., Weinstein, P. R. Effect of suture size and carotid clip application upon blood flow and infarct volume after permanent and temporary middle cerebral artery occlusion in mice. Brain Res. 970, 131-192 (2003).
- Tureyen, K., Vemuganti, R., Sailor, K. A., Dempsey, R. J. Infarct volume quantification in mouse focal cerebral ischemia: a comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J. Neurosci. Methods. 139, 203-207 (2004).

