Endurance Training Protocol and Longitudinal Performance Assays for Drosophila melanogaster
Summary
We describe the first endurance training protocol for an important genetic model species, Drosophila melanogaster, and outline several assays to chart improvements in mobility following training.
Abstract
One of the most pressing problems facing modern medical researchers is the surging levels of obesity, with the consequent increase in associated disorders such as diabetes and cardiovascular disease 1-3. An important topic of research into these associated health problems involves the role of endurance exercise as a beneficial intervention.
Exercise training is an inexpensive, non-invasive intervention with several beneficial results, including reduction in excess body fat 4, increased insulin sensitivity in skeletal muscle 5, increased anti-inflammatory and antioxidative responses 6, and improved contractile capacity in cardiomyocytes 7. Low intensity exercise is known to increase mitochondrial activity and biogenesis in humans 8 and mice, with the transcriptional coactivator PGC1-α as an important intermediate 9,10.
Despite the importance of exercise as a tool for combating several important age-related diseases, extensive longitudinal genetic studies have been impeded by the lack of an endurance training protocol for a short-lived genetic model species. The variety of genetic tools available for use with Drosophila, together with its short lifespan and inexpensive maintenance, make it an appealing model for further study of these genetic mechanisms. With this in mind we have developed a novel apparatus, known as the Power Tower, for large scale exercise-training in Drosophila melanogaster 11. The Power Tower utilizes the flies’ instinctive negative geotaxis behavior to repetitively induce rapid climbing. Each time the machine lifts, then drops, the platform of flies, the flies are induced to climb. Flies continue to respond as long as the machine is in operation or until they become too fatigued to respond. Thus, the researcher can use this machine to provide simultaneous training to large numbers of age-matched and genetically identical flies. Additionally, we describe associated assays useful to track longitudinal progress of fly cohorts during training.
Protocol
1. Power Tower Setup and Operation
- The Power Tower is placed on a rectangular piece of plywood that is clamped to a table. A 4.5 rpm gear motor from Grainger rests on top of four 2″x6″ boards and one ¾ inch plywood board. The motor, an AC/DC speed control, a fuse, and an on/off switch are wired together into a juncture box.
- To cool the motor, a hole 3 inches in diameter is cut into a vertical ½ inch piece of plywood. On one side, a 3-inch fan is attached to the plywood, and on the other, a 3-inch diameter metal cylinder is attached.
- A custom-made rotating arm with a roller mounted on the end is connected to the motor. The motor and attached arm rotate in a clockwise direction, pushing down one side of a bent ¾ inch square tube stock that is attached to the plywood with a door hinge. A wheel is attached to the top side of the tube stock below the platform to allow it to roll against the platform that is directly above it.
- The arm pushes down on one end of the tube stock and lifts the opposite end, raising a two-level platform (Figure 1D). When the roller on the arm clears the tube stock it allows the arm to return to the starting position, dropping the platform back down (Figure 1F).
- The platforms consist of racks that are placed on top of plywood and secured by bungee cords that are hooked onto screw eyes. The platforms slide up and down along drawer sliders that are attached to the plywood with brackets.
- Vials are placed into the racks and secured by a square grid screen that is held in place with bungee cords.
- A Styrofoam cushion is placed below the platform to dampen the shock of the drop.
- The power of the motor is adjusted such that a full rotational period is 15 seconds long.
2. Exercise Protocol
- Flies are housed in a 25 °C incubator with 50% humidity and a 12-hour light/dark cycle. Both exercise training and assessment of exercise capacity are conducted in a 25 °C temperature-controlled room.
- Flies are collected and age-matched within 1-2 days of each other. A minimum of 240 flies is required for longitudinal monitoring of endurance and negative geotaxis (climbing) ability, two physiological indicators that reflect the effects of exercise training. Longitudinal monitoring allows isolation of induced change relative to starting values for each cohort, without confounding effects from variation between cohorts. Flies for additional experiments should be collected as needed.
- After collection, flies are divided into 120 experimental flies, which will be subjected to the exercise regimen, and 120 control flies, which will not be exercised. The flies are stored in vials of 20 flies each. The vials of experimental flies are plugged with firm cellulose acetate Flugs and the vials of control (unexercised) flies are plugged with soft sponge stoppers, which can be pushed down to immobilize the flies while on the Power Tower. Sponge is used for immobilization because its adaptive size allows easy placement near the bottom of the vial. The softer sponge material also reduces injuries caused by the more solid Flugs.
- Flies on the Power Tower are stored and exercised in vials that contain 5 mL of food, which provides them with a softer landing than flies that are exercised in empty vials. The food is typically composed of 10% sucrose, 10% yeast, and 2% agar in water, although we do not observe flies to eat often during the course of a training session. If empty vials are used during training, the percentage of flies that suffer injuries is dramatically increased.
- The Power Tower is operated in a temperature-controlled room that is maintained at 25 °C. Experimental flies are placed on the Power Tower and made to climb. Control flies are also placed on the Power Tower, but the sponge stopper is pushed down into the vial approximately 3 mm above the flies in order to limit movement. This group serves as a control for any effects of the Power Tower that are unrelated to exercise. Although some room still exists for these flies to maneuver, we have repeatedly observed that flies under these conditions run very little and do not demonstrate a physiological response to exercise. It may also be desirable to include an additional control group that is not placed on the Power Tower at all.
- Flies are exercised five consecutive days each week, utilizing a ramped schedule (Figure 2). During week one the flies are exercised for 2 hours per session, week two for 2.5 hours per session, and week three for 3 hours per session. Although other regimens have been tested, this protocol produces consistent results across genotypes and under divergent conditions. This protocol is, however, subject to a wide array of possible variations that might suit a particular experiment.
3. Exercise and Locomotor Fatigue
- Time to fatigue during endurance exercise is a useful physiological indicator to confirm and/or quantitate exercise capacity of a given cohort. Fatigue assays can be performed longitudinally on cohorts that will be used in training experiments. Assays may be conducted either prior to training, after the completion of the training protocol, or both. Other intermediate time points can be added if charting incremental progress during training is desired. The fatigue assay can also be used to measure exercise endurance as a response to treatments other than the training protocol, such as diet alteration (Figure 3B).
- Fatigue assays should be conducted on a day when training is not taking place. Both the experimental and control flies are placed on the Power Tower in vials of 20 each and made to climb until fatigued. Fatigue is recognized as the failure to respond to a negative geotaxis stimulus with climbing behavior. Fatigue behavior is scored by visual observation.
- A vial of flies is considered “fatigued” when 5 or fewer flies are able to climb higher than 2 inches for four consecutive drops. Fatigued vials are removed from the Power Tower while it is still operating, and the time of removal is recorded. Times of removal can be plotted as a nested histogram, or as a “time-to-failure” plot. An example of data plotted as a “time-to-failure” plot is shown in Figure 3B.
- Vials are monitored at 10 minute intervals for a maximum of 10 hours (Figure 3B).
4. Exercise, Age, and Locomotor Ability
- Since this training protocol relies on induced running behavior, a primary assay for quantitating effects of training is to longitudinally measure change in average running speed during training. During the course of training, 120-fly cohorts of exercised and unexercised flies are tested daily for their response to a negative geotaxis stimulus. The RING technique described in Gargano et al. (2005) is used to measure the flies’ climbing ability 12. Briefly, this technique measures average height climbed by a vial of flies during a defined time after induction of negative geotaxis. The height climbed during a defined time is equivalent to climbing speed. Measurements are performed before placing flies on the machine for daily training so as to avoid complications from fatigue after the day’s run.
- In our standard protocol, flies are exercised for a total of three weeks and climbing ability is examined for a total of five weeks: three weeks during exercise-training and two weeks after cessation of exercise (Figure 2). This allows the experimenter to chart whether effects of exercise on mobility persist after cessation of the program.
- Four photographs of each cohort’s daily RING performance are used for the analysis of climbing ability. Six vials are included in each photograph. Negative geotaxis is induced, and using a timed camera, a photograph is taken after a defined number of seconds. We typically use two seconds, although this can be varied depending on experimental conditions. For every photograph, each vial is divided into four quadrants of equal height, and image processing software is used to assign each fly a score based on the quadrant it reached within the allotted time period. Flies that climb to the topmost quadrant receive a score of 4, flies in the next highest quadrant receive a 3, flies in the second highest quadrant receive a 2, and flies in the lowest quadrant receive a 1. Flies that do not climb off of the bottom at all are given a score of 0.
- For each vial in a given photograph, a “climbing index” is generated by averaging the scores of all the flies in that vial. Each vial’s indices from the four photographs are then averaged to give a final climbing index for that day.
- All negative geotaxis experiments are conducted longitudinally. Results are normalized to the initial “pre-exercise” score in order to reduce the effect of cohort variation in climbing ability and highlight the relative longitudinal change in a cohort’s climbing capacity as the result of exercise training. The initial score is determined by the average of the first three days’ climbing indices and is assigned a value of 1. Subsequent trials are then expressed as a percentage of the initial index.
5. Representative Results
Wild-type flies respond to an endurance protocol with a diminished age-related decrease in climbing ability that persists following the end of training, as reflected in longitudinal RING assays across five weeks of age (Figure 3A). This delayed decline in negative geotaxis is a standard phenotypic response that can serve as a positive control to ensure that wild-type exercise response is occurring normally. This data set is presented as an example of how the induction of exercise by the Power Tower program can be used as a behavioral input. The ability of various genetic or environmental factors to modulate this effect can then be assessed.
Conversely, the Power Tower can also be used as an output in various experimental designs. For example, genotype, diet, or other conditions can be varied. Then, the effect of these variations on exercise physiology can be tested using the Power Tower. Here, we show an example of this approach. When flies with a varying percentage of sucrose in their diets were tested for time to fatigue, increased sucrose content correlated with increased endurance capacity (Figure 3B).
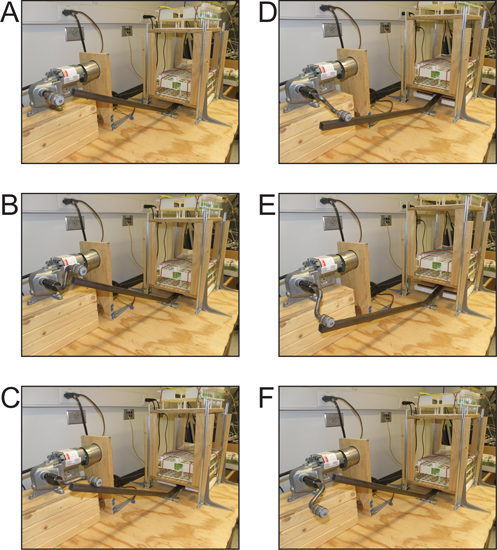
Figure 1. Operation of the Power Tower. (A-C) The motorized bent arm with attached roller rotates clockwise until it comes into contact with the bent square tube. (D,E) The arm pushes down on the bent square tube, causing the platform that is laden with vials of flies to lift. (F) As the arm clears the tube the platform is allowed to fall back down, forcing the flies to return to the bottom of the vial.
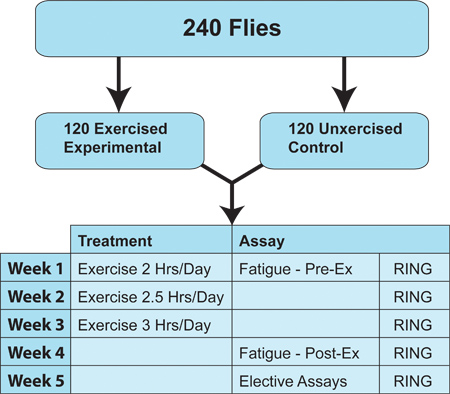
Figure 2. Suggested Exercise Protocol. Flies subjected to the training protocol are made to exercise for five days each week under a three-week long ramped regimen that progressively increases the duration of exercise from the initial 2 hours by 30 minutes each week. Standard analyses include fatigue assays before and following the exercise program and RING assays from week 1 through week 5. All assays are performed in duplicate on an equal number of unexercised flies as a negative control. Other various physiological or biochemical tests can be conducted as determined by the researcher.
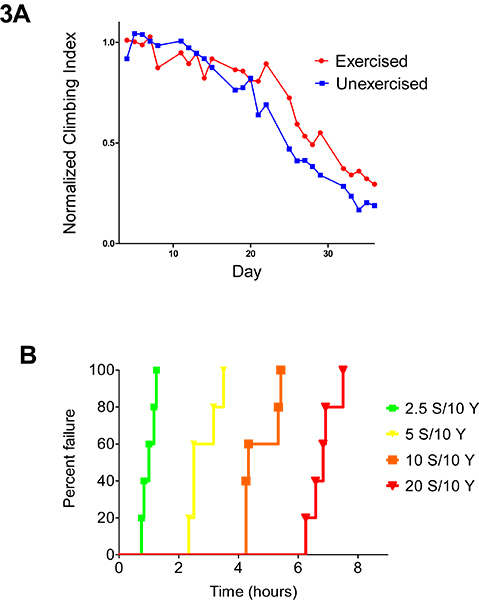
Figure 3. Endurance exercise alters multiple aspects of mobility. (A) RING assays performed longitudinally across ages in a single pair of male Y1W67C23 cohorts. Age-matched, genetically identical exercised and unexercised control flies were measured daily for average climbing speed. Results are expressed in terms of a climbing index that is normalized to the average climbing height across the first three days of measurement. Exercise-trained flies displayed a diminished age-related decline in negative geotaxis ability compared to age-matched unexercised siblings across ages (2-way ANOVA, p < 0.005). (B) Fatigue assays conducted for 8 hours on age-matched female Canton S flies show a significant effect of dietary sucrose content on time to fatigue (Log-rank, p < 0.0001). Prior to experiment, flies were fed a yeast/sucrose/agar diet, with 10% weight/volume yeast concentration, and a varying percentage of dietary sucrose. Five vials of 20 flies each were tested for each diet. Graph displays how many vials still have five or more flies running at a given time point. These results can be treated statistically and graphically as a survival (or time to failure) curve, with “failure” for a vial being defined as a point in time when less than five flies continue to respond to negative geotaxis stimulus. Note that many other possible study designs and statistical treatments are possible, and data treatment and measurement should be tailored to fit individual purposes.
Discussion
The general protocol presented here has been successful in documenting physiological effects following training. However, several areas in this protocol are subject to modification to fit particular experimental needs. For example, the length of training and number of bouts could potentially be varied to make the program more or less challenging, as desired. The height of the container in which negative geotaxis ability is measured could be altered to increase the available area for improvement to be documented. Various methods of automating quantitation of climbing speed may also be applicable. In principle, any software program capable of distinguishing a fly from background can be used to speed the data gathering process.
Some aspects of the protocol should be modified only with great caution, however. For example, preliminary experiments strongly indicate that at least one day of rest per week tends to facilitate greater improvement than relentless daily exercise. Additionally, circadian rhythms and temperature are known to affect the movement of cold-blooded animals. The time of day that training takes place can be varied, but should always be consistent within particular groups under comparison, in order to avoid the possibility of confounding effects of circadian rhythms. Temperature control is also essential, and we recommend a dedicated room at constant temperature to house exercise equipment. Lastly, males and females must be reared and measured separately, in order to avoid the potential of confounding effects of fertility and sex differences in exercise capacity.
Potential applications of this methodology are limited only by the imagination of the researcher. In preliminary work, we have utilized this methodology in three broad applications:
- To measure the effect of endurance exercise on various aspects of wild type biology across ages.
- To measure the effect of endurance exercise on specific mutant phenotypes.
- To screen for genetic factors that are necessary to execute the benefits of exercise.
Each of these applications encompasses a wide variety of specific possibilities. Based on our preliminary experience, mutant phenotypes tend to vary with exercise level as much as they vary with diet. The use of invertebrate models to better understand the relationship between diet, exercise, and aging physiology is perhaps the most important general application of this protocol.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the NHLBI to RW.
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| Dayton Gearmotor | Grainger | 1LRA6A | |
| Raco Electrical Box | Grainger | 5A052 | |
| Raco Cover | Grainger | 5A053 | |
| Cooper Bussmann Fuse | Grainger | 6F043 | |
| Cooper Bussmann Fuse Holder | Grainger | 1DD33 | |
| Carling Technologies Switch | Grainger | 2X464 | |
| Dayton Control, AC/DC Speed | Grainger | 4X796 | |
| Flugs for Narrow Plastic Vials | Genesee Scientific | 49-102 |
Riferimenti
- Wild, S., Roglic, G., Green, A., Sicree, R., King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 25, 1047-1053 (2004).
- Flegal, K. M., Carroll, M. D., Ogden, L. C., Johnson, C. L. Prevalence and Trends in Obesity Among US Adults. JAMA. 288, 1723-1727 (1999).
- Hubert, H. B., Feinleib, M., McNamar, P. M., Castelli, W. P. Obesity as an Independent Risk Factor for Cardiovascular Disease: A 26-year Follow-up of Participants in the Framingham Heart Study. Circulation. 67, 698-977 (1983).
- Ross, R., Dagnone, D., Jones, P. J. H., Smith, H., Paddags, A., Hudson, R., Janssen, I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in med – A randomized, controlled trial. Annals of Internal Medicine. 133, 92-103 (2000).
- Goodyea, L. J., Kahn, B. B. Exercise Glucose Transport, and Insulin Sensitivity. Annual Review of Medicine. 49, 235-261 (1998).
- Linke, A., Adams, V., Schulze, P. D., Erbs, S., Gielen, S., Fiehn, E., Mobius-Winkler, S., Schubert, A., Schuler, G., Hambrecht, R. Antioxidative Effects of Exercise Training in Patients With Chronic Heart Failure. Circulation. 111, 1763-1763 (2005).
- Kemi, O. J., Ellingsen, O., Smith, G. L., Wisloff, U. Exercise-induced changes in calcium handling in left ventricular cardiomyocytes. Frontiers in Bioscience. 13, 356-368 (2008).
- Wang, H., Hiatt, W. R., Barstow, T. J., Brass, E. P. Relationships between muscle mitochondrial enzyme activity and oxidative capacity in man: alterations with disease. Eur. J. Appl. Physiol. 80, 22-27 (1999).
- Wu, Z., Puigserver, P., Andersson, U., Zhang, C., Adelmant, G., Mootha, V., Troy, A., Cinti, S., Lowell, B., Scarpulla, R. C., Spiegelman, B. M. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell. 98, 115-124 (1999).
- Goto, M., Terada, S., Kato, M., Katoh, M., Yokozeki, T., Tabata, I., Shimokawa, T. cDNA Cloning and mRNA Analysis of PGC-1 in Epirtrochlearis Muscle in Swimming-Exercised Rats. Biochemical and Biophysical Research Communications. , 274-350 (2000).
- Piazza, N., Gosangi, B., Devilla, S., Arking, R., Wessells, R. Exercise-Training in Young Drosophila melanogaster Reduces Age-Related Decline in Mobility and Cardiac Performance. PLoS ONE. 4, e5886-e5886 (2009).
- Gargano, J. W., Martin, I., Bhandari, P., Grotewiel, M. S. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 40, 386-395 (2005).

