Performing Cavernous Nerve Stimulation in Rats to Measure Intracavernous Pressure
Abstract
Source: Hox, M., et al. Cavernous Nerve Stimulation and Recording of Intracavernous Pressure in a Rat. J. Vis. Exp. (2018).
This video demonstrates the isolation and stimulation of the cavernous nerve in an anesthetized rat to study blood pressure changes in the cavernous body.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of Tubing, Electrode, and Instruments for the Surgical Procedure
- Use the following microsurgical instruments: surgical scissors, angled micro scissors, tissue forceps, a pair of Dumont #7 and #5 curved micro forceps, a micro needle holder, and retractors.
NOTE: This is an acute procedure, so the instruments need not be sterilized. After use, clean and wipe the tips with 70% ethanol. - Soak the tubing in 70% ethanol and then flush it with sterile 0.9% NaCl with 100 U/mL heparin before use. Leave the tubing filled to avoid introducing air bubbles into the system.
- Cut a 20-30 cm piece of polyethylene (PE)-50 tubing to make a catheter for the intracavernous pressure (ICP) measurement. Ensure that the tubing is as short as possible to reduce dampening the pressure.
- Bend a sterile 24G needle side-to-side until the needle breaks in the middle. Connect the piece with the bevel to the distal end of the PE tubing for insertion into the penile crus. Insert the other half to the hub for connection to the pressure transducer. Fill the system with heparinized saline (100 U/mL).
- To make the bipolar Teflon-coated electrode, cut two 125 μm silver wires to equal length. Use a piece of tape to attach the wires to the edge of the table and thread them together. Then, attach the electrode to a black plate.
- Make a small incision into the Teflon and use #5 micro forceps to strip a 4-5 mm length of Teflon coating off the tips of the electrodes. Cut the tips off with a scalpel to achieve even length and create hooks by bending the ends around the blunt edge of a scalpel blade.
- Tape the electrode with the end extending slightly over the edge of the black plate with the hooks pointing upwards. Mix the silicon glue on a plastic plate for 10 s and wrap a glue bubble around the electrode 1-2 mm from the hook.
- Allow it to dry for approximately 5 min before use (Figure 1). Strip the Teflon from a longer section on the other end to allow for connection to the stimulator.
NOTE: When re-doing the hooks on the distal end, the electrode can be reused many times.
2. Preparation of the Animal
- After anesthetizing the animal, shave the lower half of the abdomen, neck, and perineum. Scrub the animal with 70% alcohol followed by povidone-iodine three times. Place the rat on a heated surgical pad in a supine position. Apply vet eye ointment and switch the anesthesia over to a nose cone with 2.5% isoflurane and 0.8 L/min oxygen as the carrier.
NOTE: Adjust the level of isoflurane and oxygen to achieve an acceptable level of anesthesia.
3. Presurgical Preparation
- Perform the entire surgical procedure under an operating microscope: a magnification ranging from 3.15X to 20X is sufficient. Use gloves and maintain a clean environment throughout the surgery. Place the rat on a drape.
4. Ischiocavernosus Muscle Dissection for the ICP Measurement
- Use a scalpel, straight scissors, and Dumont #7 curved micro forceps to make a 1 cm vertical skin incision 5 mm lateral to the midline starting at the level of the base of the penis and extending downward (Figure 2A). Use a Q-tip and carefully separate the fascia lateral to the scrotum (Figure 2B). After dissecting the fascia, attach retractors and palpate with a cotton-tipped swap to find the ischial tuberosity (Figure 2C).
- Dissect through the adipose tissue medial of this point until the ischiocavernosus muscle is visualized (Figure 3A). Use a pair of Dumont #7 curved micro forceps and longitudinally separate the muscle. The tunica albuginea will appear as a bright white structure (Figure 3B). Using micro forceps and a micro scissor, expose the tunica albuginea adequately to see its course (Figure 3C).
- After calibration of the system's settings, insert tubing through the skin on the perineum, making sure that it runs parallel to the penile crus (Figure 4). Leave the line in place and keep the incision moist with saline.
5. CN Dissection for Stimulation
- Make a 2 cm lower, midline abdominal incision through the skin using a scalpel first and then a pair of straight scissors and micro forceps. Create a matching incision through the fascia along the linea alba and the underlying muscular tissue to expose the bladder and the prostate.
- Use retractors to achieve good exposure. Use cotton-tip swabs to separate the prostate from the adipose tissue to obtain clear visualization of the major pelvic ganglion (MPG) and cavernous nerve (CN), running on the dorsolateral aspect of the prostate (Figure 5).
- After visualization of the MPG and the CN, carefully incise the fascia overlying the nerve 2-5 mm distal to the MPG with angled micro scissors (Figure 6a). With the use of #5 micro forceps, spread the tissue on each side of the nerve and underneath it to free a 4-mm long portion (Figure 6b), and slide a 9-0 suture under the nerve (Figure 6c).
- Elevate the nerve slightly with the help of the suture (Figure 7a) to facilitate placement of the hooks of the bipolar electrode around the nerve (Figure 7b). Let an assistant mix the two-component silicon glue with the tip of an insulin needle for 5 s. Dry the nerve and apply the glue to the area around the hooks and the nerve (Figure 7c, d). Keep the nerve elevated by pulling slightly on the electrode for approximately 1 min to allow the glue to dry.
- Remove the retractors, except for the retractors on the right side, to avoid any pulling or twisting of the electrode. Wet the exposed organs with saline and lay gauze soaked in saline over the incision.
6. Cavernous Body Cannulation for the ICP Measurement
- Restore visualization of the tunica albuginea using retractors. Make sure not to attach the retractors to the ischiocavernosus muscle as it will distort the crus.
- Attach the needle and flush the tubing with heparinized saline before introducing it into the tunica albuginea. Keep the tunica albuginea stretched, using Dumont #7 curved micro forceps in one hand (non-dominant), holding the tunica albuginea and the rest of the overlying muscle distal to the insertion point. Hold the needle with straight micro forceps in the other dominant hand and introduce it parallel with the course of the cavernous body (Figure 8).
- Push the needle 5-8 mm into the cavernous body. Flush the tubing and press on the crus to test the line (Figure 9). Ensure that there are no leaks. Fasten the tubing to the table with tape to prevent accidental pulling on the line. Remove the retractors.
7. Stimulation of the CN
- When running the recording program (e.g., Spike 2), continuously record the intracavernous and mean arterial pressure.
- Set the following parameters on a stimulator (e.g., SD9 Grass Instruments, see Table of Materials) for CN stimulation: current at 1.5 mA, frequency at 16 Hz, voltage at 3 V, and pulse width at 5 ms. Apply 50 s of stimulation with a minimum of 1 min of rest between stimulations.
NOTE: The first stimulations usually result in a diminished response (Figure 10).
Representative Results
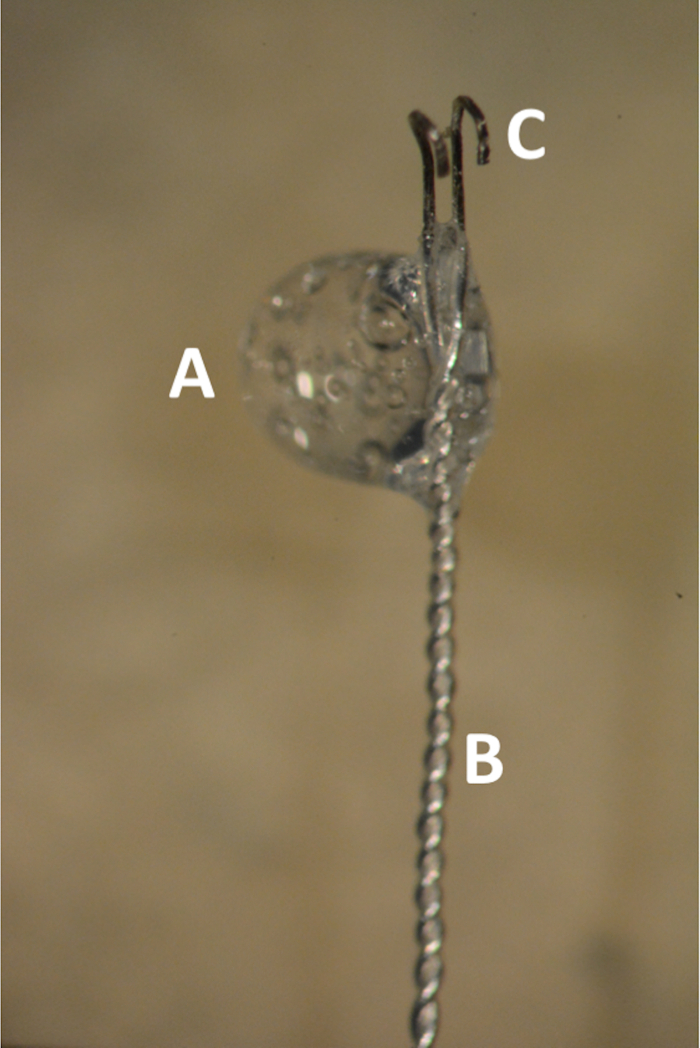
Figure 1. The bipolar Teflon coated silver electrode. (A) Glue bubble in the transition zone between the coated and uncoated electrode. (B) Distal 2 cm of the electrode tightly braded. (C) Parallel uncoated hooks 1 mm apart.
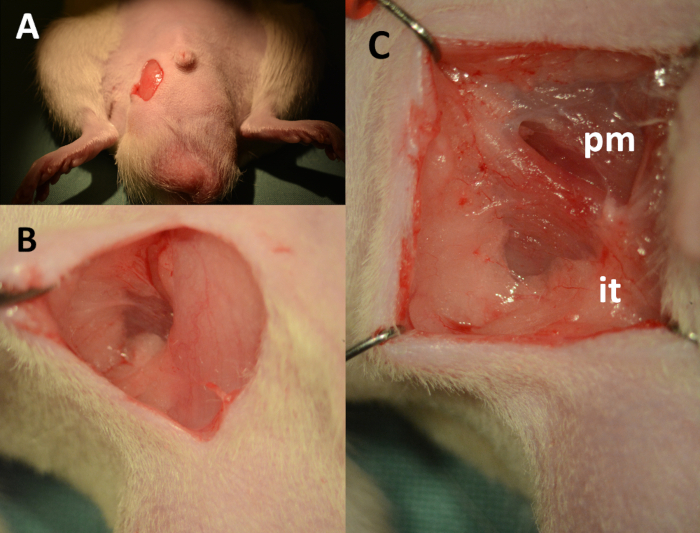
Figure 2. Dissection of the cavernous body – landmarks. (A) 1 cm vertical skin incision, downward starting 2 mm lateral from the base of the penis. (B) Fascia lateral to the scrotum separated using cotton-tipped swabs. (C) View of the operating field after placement of retractors (pm: pyramidalis muscle, it: insertion point of crus to the ischial tuberosity).
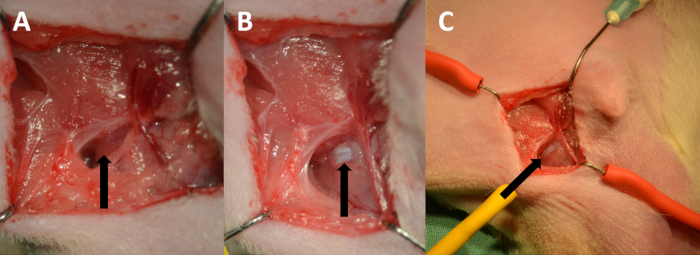
Figure 3. Exposing the tunica albuginea. (A) The ischiocavernosus muscle (arrow). (B) Tunica albuginea (arrow). (C) Low power magnification showing the course of the cavernous body.
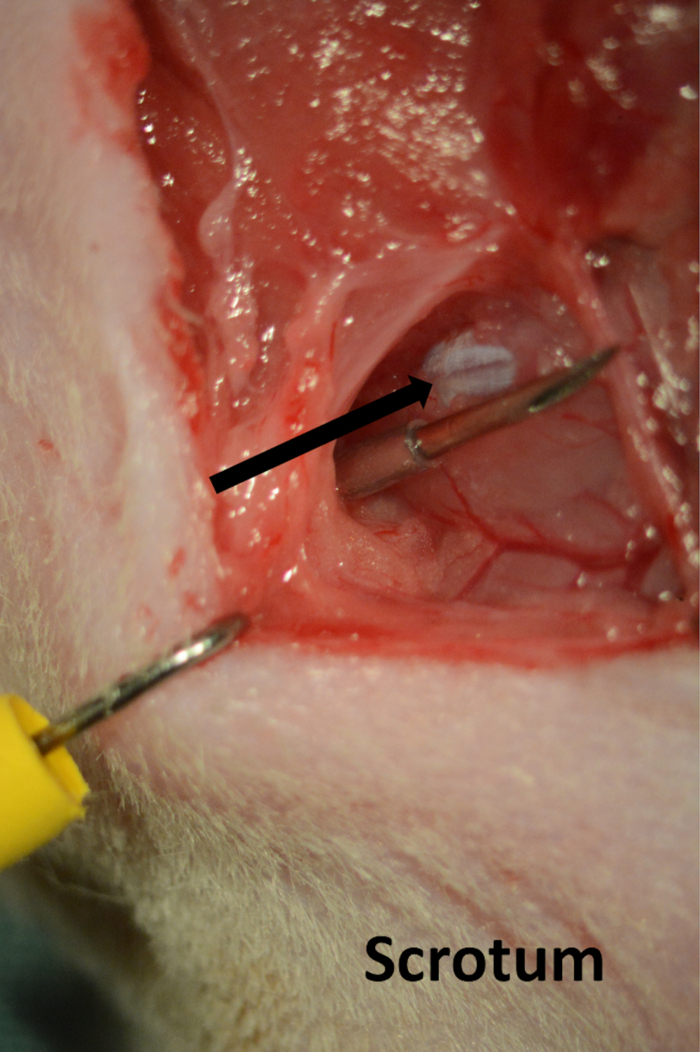
Figure 4. Line for cavernous pressure recording. Needle introduced through the skin on the perineum running parallel with cavernous body(arrow).
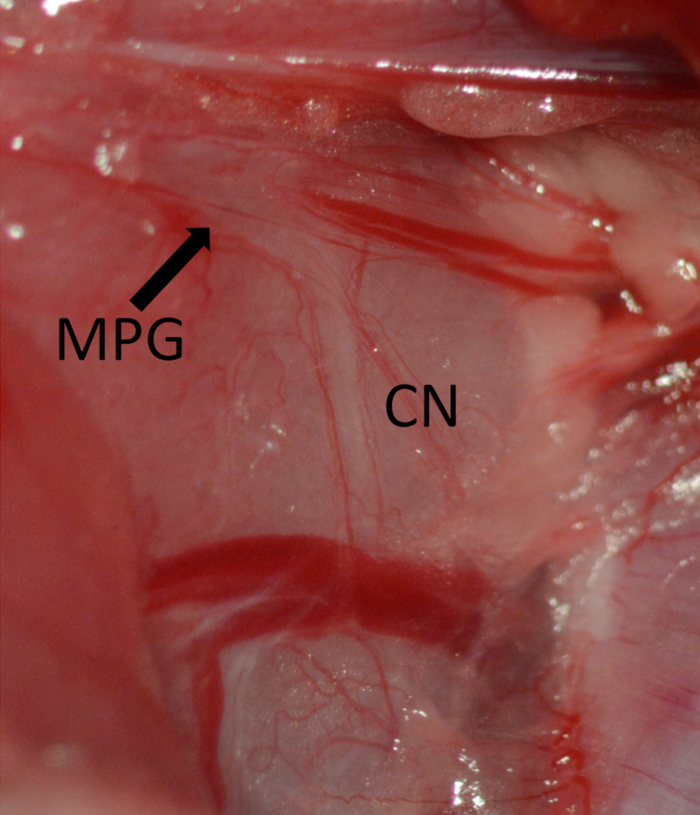
Figure 5. Exposure of the MPG marked by arrow and CN running vertical on the dorsolateral aspect of the prostate. Major pelvic ganglion (MPG) marked by arrow, cavernous nerve (CN).
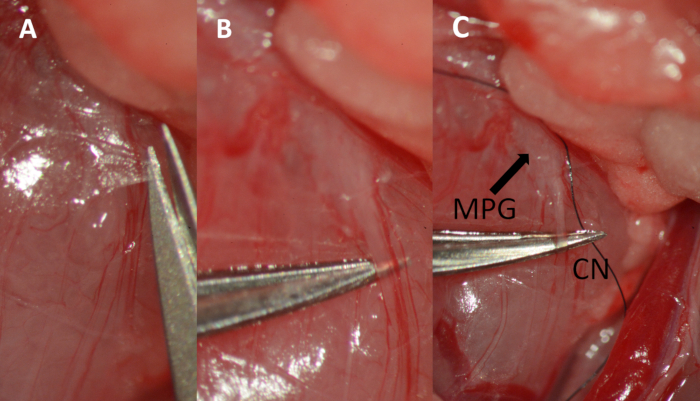
Figure 6. Dissection of the cavernous nerve. (A) Cutting the fascia overlying the cavernous nerve with micro scissors. (B) Separating the nerve from the underlying tissue using micro forceps. (C) Placing a 9-0 ligature underneath the nerve. Major pelvic ganglion (MPG) marked by arrow, cavernous nerve (CN).
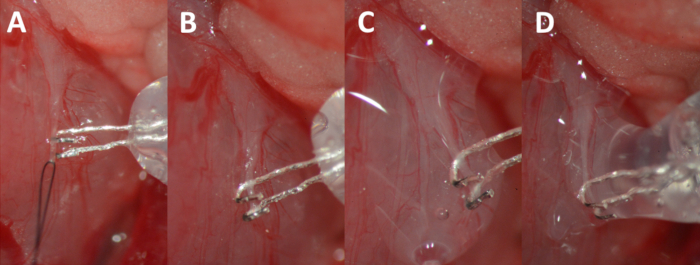
Figure 7. Hooking of the nerve. (A) Elevating the nerve by gently pulling on the suture. (B) Nerve resting in the hooks of the electrode. (C) Nerve and electrode complex isolated with the biocompatible silicon glue. (D) Additional glue bubble added to completely isolate the nerve-electrode complex.
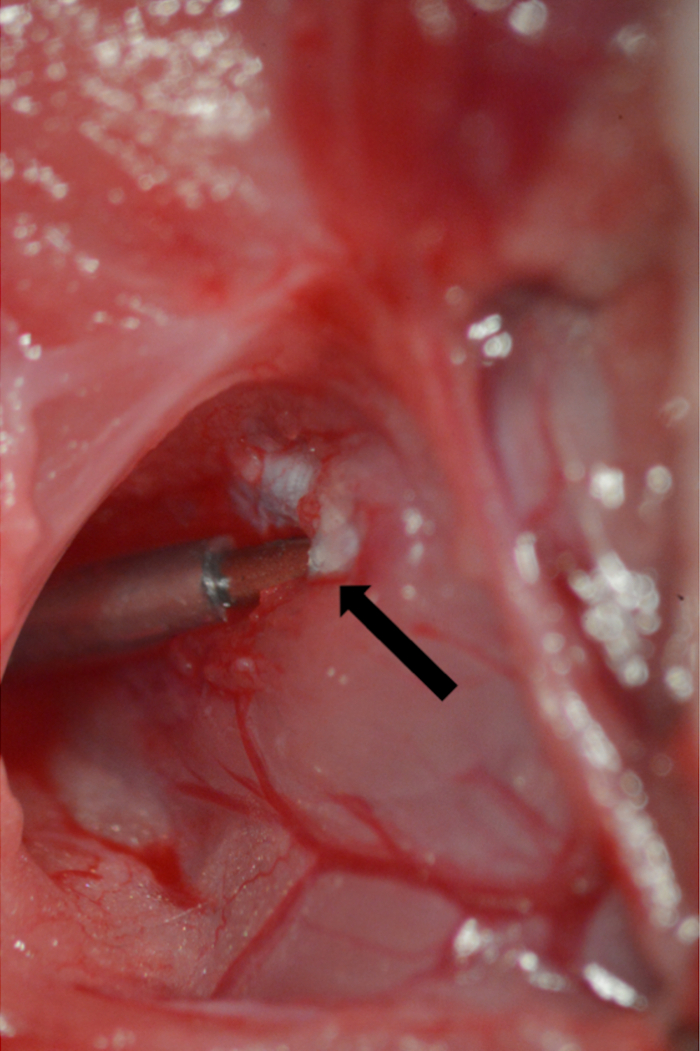
Figure 8. Cannulation of the tunica albuginea. A 23 G needle connected to PE-50 tubing inserted into the tunica albuginea. Point of insertion marked by arrow.
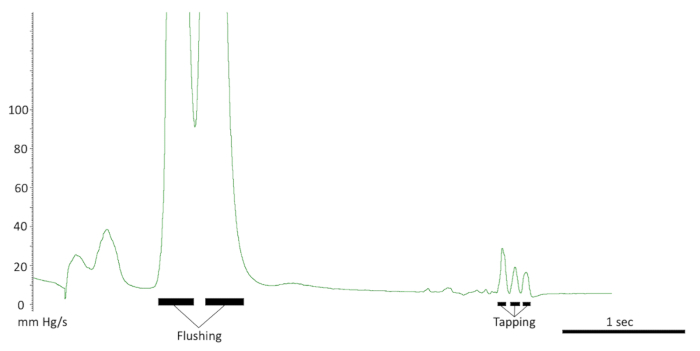
Figure 9. Testing the intracavernous line. The responses seen with a correct line placement. Note the flushing of the line and response to tapping on the crus. Also, note the quick pressure drop back to baseline.
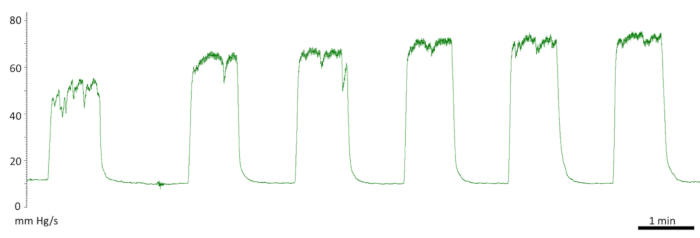
Figure 10. Initial responses to cavernous nerve stimulation. Diminished first response. First stimulation of 50 mm Hg and a fluctuating plateau. Second and third stimulation of 66 mm Hg. The following measurements were recorded at the normal level at 73 mm Hg.
Divulgazioni
The authors have nothing to disclose.
Materials
| Adson forceps | Fine Science Tool | 11006-12 | |
| Dumont #7 forceps | Fine Science Tool | 11271-30 | |
| Dumont #5 forceps | Fine Science Tool | 11273-20 | |
| ToughCut Mayo scissor | Fine Science Tool | 14110-15 | |
| Miniature Vannas Spring scissor | Fine Science Tool | 15006-09 | |
| Ultra Fine Hemostat | Fine Science Tool | 13020-12 | |
| Crile Hemostat | Fine Science Tool | 13004-14 | |
| Kwik-Sil Adhesive | World Precision Instruments | KWIK-SIL | |
| Teflon coated silver wire 0.125 mm | World Precision Instruments | AGT0510 | |
| Elastic wire retractors | Custom made | ||
| Scalpel blade | Fine Science Tool | 10023-00 | |
| PE-50 tubing | Warner Instruments | 64-0753 | |
| 23 G Needle | Kruuse | 121272 | |
| SD-9 Square Pulse Stimulator | Somatco | 1077/183 | |
| Blood pressure transducer and cable | World Precision Instruments | BLPR2 | |
| Raucotupf Cotton-tipped Applicators | Lohmann-Raucher | 11966 | |
| Pro-ophta Ocular Sticks | Lohmann-Raucher | 16515 | |
| NaCl 0,9 % 100 mL | Local pharmacy | ||
| Heparin | Local pharmacy | ||
| 25 mL Syringe | Odense University Hospital | ||
| Vet eye ointment – Viscotears | Local pharmacy | ||
| silver wires | Science Products GmbH, Heidelberg, Germany | ||
| Silicon Glue | Kwik-Sil, World Precision Instruments, Sarasota, FL, USA |

