Preparing Cerebral Endothelial Tubes from Mouse Parenchymal Arterioles
Abstract
Source: Hakim, M. A., et al., Isolation and Functional Analysis of Arteriolar Endothelium of Mouse Brain Parenchyma. J. Vis. Exp. (2022)
This video demonstrates a method for isolating the endothelial tubes from mouse cerebral parenchyma. Through a combination of enzymatic digestion and mechanical dissociation, the process yields an intact tube with endothelial cells.
Protocol
All procedures involving animal samples have been reviewed and approved by the appropriate animal ethical review committee.
1. Solutions and drugs
- Physiological salt solution (PSS)
- Prepare a minimum of 1 L of PSS using 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES), and 10 mM glucose.
- Prepare necessary solutions lacking CaCl2 (zero Ca2+ PSS) for dissection of cerebral arterioles and isolation of endothelial tube.
NOTE: Prepare all solutions in ultrapure deionized H2O, followed by filtration (0.22 µm). Ensure that the final product contains a pH 7.4 with osmolality between 290 and 300 mOsm.
- Bovine serum albumin (BSA, 10%)
- Dissolve 1 g of the lyophilized BSA powder in a beaker of 10 mL of zero Ca2+ PSS. Cover the beaker and allow at least 2 h with slow stirring for the BSA to dissolve.
- Filter solution using a 10 mL syringe plus a 0.22 µm filter and prepare 1 mL aliquots to be stored at -20 °C.
- Dissection solution
- Add 500 µL of BSA (10%) to 49.5 mL of zero Ca2+ PSS for a total volume of 50 mL.
- Transfer the solution to a Petri dish for arteriolar dissections.
- Dissociation solution
- Add 5 µL of CaCl2 solution (1 M) and 500 µL of BSA (10%) to 49.5 mL of zero Ca2+ PSS for a total volume of 50 mL. Incubate the solution at room temperature for at least 1 h.
- Enzymes
- Prepare 1 mL of digestion solution with 100 µg/mL papain, 170 µg/mL dithioerythritol, 250 µg/mL collagenase (Type H blend), and 40 µg/mL elastase.
NOTE: Enzyme activities can vary, although successful protocols will likely require the following enzymatic activities: ≥ 10 units/mg for papain, ≥ 1 unit/mg for collagenase, and ≥ 5 units/mg for elastase.
- Prepare 1 mL of digestion solution with 100 µg/mL papain, 170 µg/mL dithioerythritol, 250 µg/mL collagenase (Type H blend), and 40 µg/mL elastase.
2. Isolation of parenchymal arterioles
NOTE: Arterioles arising from the middle cerebral arteries (MCAs) and embedded in brain parenchyma are chosen for this protocol. The arterioles are isolated as previously described11, with modification.
- Use steel pins (diameter: 0.2 mm, length: 11 to 12 mm) to secure the isolated brain in cold dissection solution in a Petri dish containing a charcoal-infused silicon polymer coating (depth ≥ 50 cm).
- Using sharp and aligned dissection scissors, cut a rectangle of brain tissue (length: 5 mm, width: 3 mm) (Figure 1B) around the MCA while ensuring that the upper part of the tissue segment is past the branching point from the Circle of Willis. Cut another rectangle of brain tissue from the other hemisphere of the brain to access more arterioles if necessary.
- Secure the separated brain tissue segment into the dish with the MCA facing upwards (distal from the Circle of Willis) using steel pins (diameter: 0.1 mm, length: 13 to 14 mm). Carefully make a shallow incision near the pins to remove the pia with small forceps, gently peeling from one end towards the other (Figure 1C). Remove the pia from the other tissue segment to access more arterioles if needed. Carefully secure the isolated pia with parenchymal arterioles branched from MCA in the dish with the pins, and dissect the parenchymal arterioles (Figure 1D), ensuring that the arterioles are not damaged.
NOTE: If the arterioles are not easily pulled out with the pia from the parenchyma, use fine forceps and dig into the brain, starting at the MCA branch point from the Circle of Willis. Locate the arteriole, carefully loosen the tissue around arterioles (Figure 1E), and gently pull the arteriole out of the parenchyma, holding the upper end of the arteriole (Figure 1F). Multiple arterioles (length: ~1.5-2 mm) can be isolated from one rectangle of brain tissue. - Ensure the arteriole is clean with no tissue attached to it, and cut off any remaining distal branches (Figure 2A). Use this clean, intact arteriole for enzymatic digestion. Alternatively, cut each arteriole into two pieces (length: 0.75-1 mm) for enzymatic digestion for the preparation of endothelial tubes if desired.
3. Digestion of parenchymal arterioles and preparation of endothelial tubes
- Arrangement of equipment and pipettes
NOTE: Isolation of arterial endothelial tubes from the brain has been described previously13,18. The current protocol incorporates modifications for the isolation of endothelium from parenchymal (intracerebral) arterioles. Multiple arterioles or/and pieces of arterioles can be used together for enzymatic digestion.- Assemble the trituration apparatus13 for preparing endothelial tubes using an aluminum stage supporting a chamber and micromanipulators. Assemble a microscope equipped with objectives (5x-60x) and a camera connected to a computer monitor. Secure a microsyringe with a pump controller next to the stage and specimen.
- Secure a trituration pipette backfilled with mineral oil over the microsyringe piston. Use the microsyringe with the pump controller to withdraw the dissociation solution into the trituration pipette (~130 nL) on top of the mineral oil while taking care to avoid air bubbles in the pipette.
- Partial digestion of arteriolar segments
- Place intact arteriolar segments into 1 mL of dissociation solution in a 10 mL glass tube containing 100 µg/mL papain, 170 µg/mL dithioerythritol, 250 µg/mL collagenase, and 40 µg/mL elastase. Incubate arteriolar segments at 34 °C for 10-12 min.
- After digestion, replace the enzyme solution with 3-4 mL of fresh dissociation solution at room temperature.
- Isolation of arteriolar endothelial tube
NOTE: Following digestion and replacement of the enzyme solution, transfer one or multiple partially digested segments into the dissociation solution in a superfusion chamber attached to a mobile platform. While viewing at 100x to 200x magnification, select one partially digested but unbroken vessel segment and focus on it under the microscope.- Place the trituration pipette attached to the microsyringe injector in the dissociation solution in the chamber and position it close to one end of the digested vessel segment. Set a rate within the range of 1-3 nL/s on the pump controller for gentle trituration.
- While maintaining 100x to 200x magnification, withdraw the arteriolar segment into the pipette and then eject to dissociate the adventitia and smooth muscle cells (Figure 1B). Triturate the vessel segment until all smooth muscle cells are dissociated, and only endothelial cells remain as an intact "tube."
NOTE: If necessary during trituration, carefully use fine-tipped forceps to remove the dissociated adventitia and internal elastic lamina from the endothelial tube. Typically, only a few cycles of trituration yield an intact endothelial tube. - Use micromanipulators to secure each end of the endothelial tube on the glass coverslip in the chamber with borosilicate glass pinning pipettes (Figure 2C,D).
- Securing the arteriolar endothelial tube
- Replace the dissociation solution with superfusion solution (PSS containing 2 mM CaCl2) while ensuring that nonendothelial material is washed out of the chamber.
- Transfer the secured endothelial tube in the mobile platform to the microscope rig designed for continuous superfusion and intracellular or fluorescent recordings/imaging.
- Apply continuous delivery of PSS during the experiment. Manually set the flow rate (5-7 mL/min) throughout the experiment using the inline flow control valve, consistent with the laminar flow, while matching the feed of solution flow to the extent of vacuum suction. Allow ≥5 min of PSS flow to the chamber for superfusion of the endothelial tube before acquiring background data and/or dye loading.
Representative Results
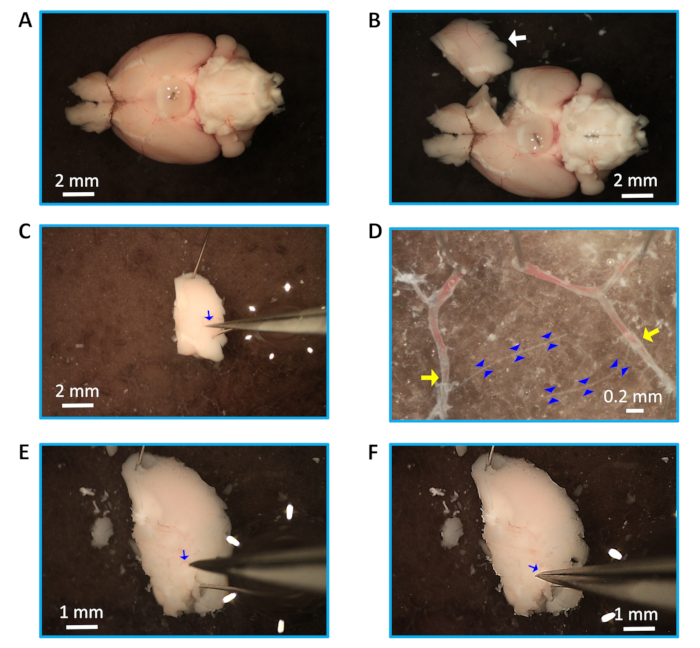
Figure 1: Dissection and isolation of parenchymal arterioles. (A) Isolated mouse brain with intact Circle of Willis. (B) A dissected rectangular piece of brain tissue containing the MCA (white arrow). (C) Isolation of pia from the upper part of the tissue (arteriole, blue arrow). (D) Arterioles (outlined with blue arrowheads) connected to middle cerebral arteries (yellow arrows). (E) Identified arteriole (blue arrow) in the brain tissue. (F) Pulling out the arteriole (blue arrow) from the loosened tissue. Note that panels E and F indicate an alternative method for isolation of the arteriole (see protocol step 3.2.3). Scale bars = 2 mm (A,B,C), 0.2 mm (D), and 1 mm (E,F).
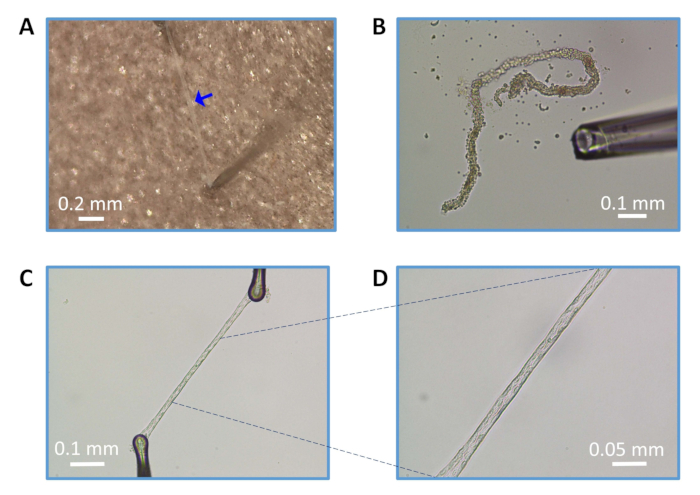
Figure 2: Preparation of endothelial tubes from parenchymal arterioles. (A) Isolated and clean arteriole (blue arrow). (B) Trituration of partially enzyme-digested arteriole. (C) Intact endothelial tube (magnification: 100x) prepared and secured on the glass coverslip using pinning pipettes. (D) Endothelial tube at 200x magnification. Scale bars = 0.2 mm (A), 0.1 mm (B, C), and 0.05 mm (D).
Divulgazioni
The authors have nothing to disclose.
Materials
| Bath Chiller (Isotemp 500LCU) | ThermoFisher Scientific | 13874647 | |
| Borosilicate glass capillaries (Pinning) | Warner Instruments | G150T-6 | |
| Borosilicate glass capillaries (Sharp Electrodes) | Warner Instruments | GC100F-10 | |
| Borosilicate glass capillaries (Trituration) | World Precision Instruments (WPI), Sarasota, FL, USA | 1B100-4 | |
| BSA: Bovine Serum Albumin | Sigma | A7906 | |
| CaCl2: Calcium Chloride | Sigma | 223506 | |
| Collagenase (Type H Blend) | Sigma | C8051 | |
| Data Acquision Digitizer | Molecular Devices, Sunnyvale, CA, USA | Digidata 1550A | |
| Dissection Dish (Glass Petri with Charcoal Sylgard bottom) | Living Systems Instrumentation, St. Albans City, VT, USA | DD-90-S-BLK | |
| Dithioerythritol | Sigma | D8255 | |
| DMSO: Dimethyl Sulfoxide | Sigma | D8418 | |
| Elastase (porcine pancreas) | Sigma | E7885 | |
| Flow Control Valve | Warner Instruments | FR-50 | |
| Fluorescence system interface, ARC lamp & power supply, hyperswitch and PMT | Molecular Devices, Sunnyvale, CA, USA | IonOptix Systems | |
| Forceps (Fine-tipped, sharpened) | FST | Dumont #5 & Dumont #55 | |
| Glucose | Sigma-Aldrich (St. Louis, MO, USA) | G7021 | |
| HEPES: (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) | Sigma | H4034 | |
| KCl: Potassium Chloride | Sigma | P9541 | |
| MgCl2: Magnesium Chloride | Sigma | M2670 | |
| Micromanipulator | Siskiyou | MX10 | |
| Micropipette puller (digital) | Sutter Instruments, Novato, CA, USA | P-97 or P-1000 | |
| Microscope (Nikon-inverted) | Nikon Instruments Inc, Melville, NY, USA | Ts2 | |
| Microscope (Nikon-inverted) | Nikon Instruments Inc | Eclipse TS100 | |
| Microscope Stage (Aluminum) | Siskiyou, Grants Pass, OR, USA | 8090P | |
| Microsyringe Pump Controller | World Precision Instruments (WPI), Sarasota, FL, USA | SYS-MICRO4 | |
| NaCl: Sodium Chloride | Sigma | S7653 | |
| Papain | Sigma | P4762 | |
| Phase contrast objectives | Nikon Instruments Inc | (Ph1 DL; 10X & 20X) | |
| Scissors (3 mm & 7 mm blades) | Fine Science Tools (or FST), Foster City, CA, USA | Moria MC52 & 15000-00 | |
| Scissors (Vannas style; 9.5 mm & 3 mm blades) | World Precision Instruments | 555640S, 14364 | |
| Temperature Controller (Dual Channel) | Warner Instruments | TC-344B or C | |
| Valve Control System | Warner Instruments | VC-6 |

