Co-Culture of Schwann Cells with DRG Neurons on a Pre-Stretched Membrane
Abstract
Source: Liu, C., et al. An Approach to Enhance Alignment and Myelination of Dorsal Root Ganglion Neurons. J. Vis. Exp. (2016).
The video demonstrates the co-culturing of dorsal root ganglion (DRG) neurons with Schwann cells. DRG neurons are first grown on a pre-stretched membrane for aligned axon growth. Schwann cells are then introduced, which attach to the axons, insulating them and promoting neural cell survival and function.
Protocol
1. Culture of dorsal root ganglion (DRG) on Pre-stretched Surface
- Preparation of Pre-stretched Anisotropic Surface
- Mix a 10:1 solution of base and curing agent and pour the mixture into a tissue culture dish (12 cm diameter). Use 4,900 mg base and 490 mg curing agent for the total crosslinking mixture.
- Keep the gel mixture under vacuum for 20 min to remove air bubbles.
- Place the gel mixture in the oven for overnight curing at 60 °C.
- After the poly-dimethyl-siloxane (PDMS) membrane cures, treat the surface with oxygen plasma using a plasma cleaning/etching system for 3 min at 165 mTorr and 65 sccm flow of O2.
- Cut a piece of rectangular membrane (5 × 3.5 cm) from the dish; while being cognizant of which side is oxygen-treated, make sure the oxygen-treated side is facing up and fix it onto a stretching frame. Place the frame on a stretching stage and evenly stretch by turning the knob on the stage until it reaches 10% elongation (or some other pre-determined stretch; the elongation can be directly read from the stretching stage) in the longer axis. Fix the stretch by tightening the screws on the frame.
- Remove the frame from the stage. Ensure the membrane surface is dry and free of dust, then place a silicone chamber onto the membrane, allowing the sticky side of the chamber to attach tightly to the membrane.
Note: The silicone chamber provides a well for retaining the cell medium on the PDMS membrane. The device design is shown in Figure 1. - Sterilize the surface with UV for 10 min.
- Add 1 ml Poly-L-Lysine (PLL) (0.01% in Phosphate Buffered Saline) to the PDMS surface within the chamber within 6 hr of plasma treatment and incubate for 2 hr at 37 °C prior to seeding the DRG neurons. This enhances cell attachment.
- Prior to seeding the cells, remove the PLL solutions from the chamber and rinse the PDMS surface with sterile water and air dry.
- Take a suspension of the DRG cells. Let the suspension set for 1 – 2 min to allow the debris to settle to the bottom of the tube. Add 1.5 ml of cell suspension into each stretch chamber and incubate at 37 °C at 5% CO2.
- To eliminate glial cells, at 1 day in vitro (DIV), add 10 µl (or 15 µl) mixture of fluoro-2 deoxy-uridine and uridine (FDU-U) stock solution (See Table of Materials) to each well. After 7 hr, replace this medium with fresh standard growth medium and place the pre-stretched culture device in a 37 °C incubator with 5% CO2.
- During the cell culture period (2 – 3 weeks), change the media every two days by replacing half the spent medium with fresh medium.
Note: After culturing on the stretched and unstretched surfaces for 2 weeks, purified SCs are added to the chamber and cultured for another week (step 2.7).
2. Co-culture of Schwann Cells (SCs) with DRG Neurons on Pre-stretched Surface
- Remove the culture medium from the flak containing SCs, and add 5 ml 0.05% Trypsin-EDTA into the flask. Incubate the cells at 37 °C at 5% CO2 for 2 – 3 min.
- Check cells under the optical microscope using a 10X objective to see if they lift up from the flask, then add 5 ml of culture medium and mix with the cells in the flask.
- Add the cell suspension to a 15 ml centrifuge tube and centrifuge at 200 x g and 20 °C for 5 min.
- Remove the supernatant and resuspend the pellet in 7 ml standard DRG growth media.
- Take 10 µl of cell suspension into a 0.5 ml microcentrifuge tube, mix with 10 µl of trypan blue, and then count the cell number using a hemocytometer under optical microscopy using a 10X objective. Dilute the cell suspension to 5,000 cells per ml by adding DRG growth media.
- Remove 0.5 ml of medium from the DRG culture in the stretched chamber and add 0.5 ml of SC suspension to the DRG culture.
- Culture the cells for 1 week at 37 °C and 5% CO2. Change the media every two days by replacing half the spent medium with fresh standard growth medium for DRG for 1 week.
Representative Results
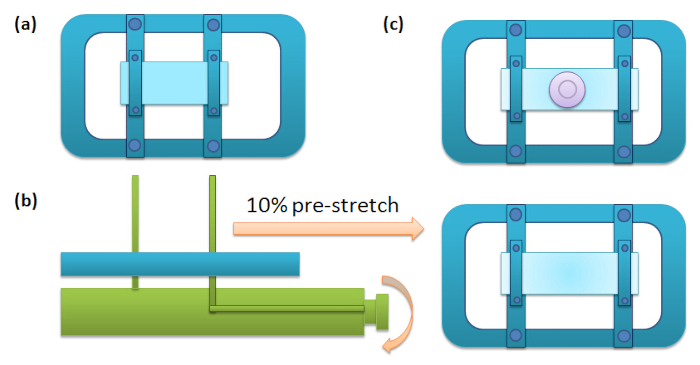
Figure 1: Schematic for the Pre-stretch Procedure. (a) A piece of PDMS membrane is clipped onto two strips of the frame that are slidable. The frame of the device is approximately 7" x 5"; the braces are about 5" x 1; the clamps on the braces are about 3" x .25", and the cup is about 1.5" diameter wide open top, 1" open bottom, and about 0.25" tall. (b) Place the frame onto the stretching stage, turn the roller to apply 10% elongation, and then fix the positions of the strips by tightening the screws. (c) Remove the frame from the stretching stage, and attach a silicon chamber onto the membrane.
Divulgazioni
The authors have nothing to disclose.
Materials
| Polydimethylsiloxane (PDMS) | Dow Corning | SYLGARD 184 | |
| Neurobasal Medium 1X | GibcoBRL | 21103-049 | |
| B27 Supplement 50X | GibcoBRL | 17504-044 | |
| Glutamax-I 100X | GibcoBRL | 35050-061 | |
| Albumax-I | GibcoBRL | 11020-021 | |
| Nerve Growth Factor-7S | Invitrogen | 13290-010 | |
| Penicillin-streptomycin | GibcoBRL | 15140-122 | |
| 0.05% Trypsin-EDTA/1mM EDTA | GibcoBRL | 25300-054 | |
| Poly-L-Lysine | Trevigen | 3438-100-01 | |
| Poly-D-Lysine | Sigma | p-6407 | |
| Fluoro-2 deoxy-uridine | Sigma | F0503 | |
| Uridine | Sigma | U3003 | |
| Hank's Balanced Salt Solution (HBSS) | Invitrogen | 14170-112 | Isolation Buffer |
| Type I Collagenase | Worthington | LS004196 | |
| Silicone chamber | Greiner bio-one | FlexiPERM ConA | |
| Plasma cleaning/etching system | March Instruments | PX-250 | |
| Standard growth medium | For 500 ml Neurobasal Medium 1X, add 10 ml of B-27 50X, 5 ml of Glutamax-I 100X, 2.5 ml of Penicillin/Streptomycin (Penn/Strep), 1 ml of Albumax-I, and 1 μl of NGF– 7S (50 ug/ml). | ||
| FDU and Uridine stock solution | FDU 100mg in 10ml of ddw (10mg/ml), filter in the hood and divided in 500ul aliquots and store at -20 ºC. | ||
| Uridine 5g in 166.7ml of ddw (33mg/ml), filter in hood, divide in 200ul aliquots and store at -20 ºC. | |||
| Take 61.5ul of FDU (10mg/ml) and 20.5ul of Uridine(33mg/ml), and add 4918ul of ddw to a final stock concentration, then divide in 1 ml aliquots and store at -20 ºC. |

