Generating Neurons and Glial Cells from Human Induced Pluripotent Stem Cell-Derived Embryoid Bodies
Abstract
Source: Pistollato, F., et al. Protocol for the Differentiation of Human Induced Pluripotent Stem Cells into Mixed Cultures of Neurons and Glia for Neurotoxicity Testing. J. Vis. Exp.(2017)
This video demonstrates the process of differentiating embryoid bodies (EBs) into neuroepithelial aggregates and their subsequent division into neuronal and glial cells. This method allows the efficient generation of neuronal and glial cells in vitro, which serve as test systems for toxicity testing as well as assessment of different pathways involved in neurotoxicity.
Protocol
1. HiPSC Differentiation into Mixed Neurons and Glia
NOTE: The procedure takes approximately 28 days to complete, with the main steps outlined in Figure 1.
- Generation of embryoid bodies (EBs) (Days 0 → 1)
NOTE: This procedure requires good manual skills and precision. HiPSC (human induced pluripotent stem cells) colony fragments should be of equal size to obtain homogenous embryoid bodies (EBs) in the next steps. Morphologically differentiated colonies (with large cytoplasmic fractions and small nucleoli) should be discarded.- Refresh the hiPSC medium (3 mL/60-mm Petri dish) prior to cutting the undifferentiated hiPSC colonies (about 1 mm in diameter, see Figure 2A) under sterile conditions.
- Cut the undifferentiated colonies (as shown in Figure 2A and Figure 2B) into fragments of approximately 200 µm x 200 µm using a 1-mL syringe with a 30G needle. Use a stereoscopic microscope at 4X magnification in a laminar flow cabinet at room temperature.
- Detach the colony fragments from the dish surface using a 200-µL pipette by gently pipetting medium underneath to lift the pieces.
- Transfer all detached fragments and the medium to a 15-mL tube using a 1, 2, or 5 mL pipette.
- Rinse the dish with 2 mL of complete hiPSC medium to recover all fragments.
- Centrifuge at 112 x g for 1 min.
- Aspirate the supernatant and gently resuspend the fragments in 5 mL of complete hiPSC EB medium (see the Table of Materials).
- Plate the colony fragments in a 60 mm ultra-low attachment Petri dish (5 mL/60-mm Petri dish).
- Incubate the Petri dish overnight at 37 °C and 5% CO2.
- On the next day (Day 1), collect the EBs and their medium in a 15 mL tube using a 1, 2, or 5 mL pipette.
- Centrifuge the EBs at 112 x g for 1 min.
- Carefully aspirate the supernatant and gently resuspend the EBs in 5 mL of complete hiPSC EB medium using a 1, 2, or 5 mL pipette.
- Replate the EBs onto a new 60-mm ultra-low attachment Petri dish (5 mL/60 mm Petri dish).
- Incubate the Petri dish overnight at 37 °C and 5% CO2.
- On Day 1, coat the dishes with basement membrane matrix (e.g., matrigel, referred to hereafter as "standard matrix") or any other suitable protein substrate (e.g., laminin).
- Store the standard matrix (see the Table of Materials) at -80 °C in 200-µL aliquots using cold 1.5 mL tubes and cold 5 or 10 mL pipettes.
- Thaw 200 µL of standard matrix on ice.
- Dilute 200 µL of standard matrix in 20 mL of basal (DMEM/F12) medium (1:100 dilution).
- Coat 60-mm Petri dishes with this solution (5 mL/dish).
- Incubate the coated dishes at 37 °C overnight.
NOTE: These dishes will be used to plate the EBs (about 50 EBs/dish) and generate neuroepithelial aggregates (rosettes); see step 1.3.
- Generation of neuroepithelial aggregates (rosettes) (Days 2 → 7)
- On Day 2, remove the standard matrix coating solution from the 60-mm Petri dishes (no need to rinse the plates) and fill them with 5 mL/dish of complete neuroepithelial induction medium (NRI); see the Table of Materials.
- Transfer the floating EBs (from step 1.1.14) to coated dishes (~50 EBs/dish) using a 200 µL pipette under a stereoscopic microscope at 4x magnification and placed in a laminar flow cabinet.
NOTE: It is critical to select homogenous, medium-sized EBs (~200-300 µm in diameter). Too-small EBs may not survive well during neuroectodermal differentiation, while too-large EBs tend to undergo core necrosis. - Incubate the dishes at 37 °C and 5% CO2.
- The day after (Day 3), check the dishes under the microscope at 10x magnification to ensure that the EBs are all attached.
- Gently perform a total medium change with a complete NRI medium.
- Change the NRI medium every other day up to day 7, when neuroepithelial aggregates (rosettes) should be visible.
- On Day 7, coat standard matrix (or laminin), as described in step 1.2, onto any required plate or dish format: 96-well plates (100 µL/well), 24-well plates (250 µL/well), 12-well plates (500 µL/well), MEA chips (for electrical activity, 1 mL/single-well chip), or 60 mm Petri dishes (4 mL/dish).
- Incubate the coated plates/dishes for at least 2 h at 37 °C and 5% CO2.
- Rosette dissociation and neuronal differentiation (Days 8 → 28)
NOTE: This procedure requires good manual skills and precision. To avoid collecting mesodermal and endodermal cells, only ectoderm rosette-like structures should be dissociated and collected.- On Day 8, cut the rosette-like structures into fragments under a stereoscopic microscope at 10X magnification in sterile conditions. Use a 1 mL syringe with a 30G needle. Note that the rosettes tend to detach from the dish when touched with the needle easily.
- Complete the detachment of the rosette fragments using a 200-µL pipette.
- Transfer the dish under the laminar flow hood and collect the rosette fragments and their medium into a 15 mL conical tube using a 1, 2, or 5 mL pipette. Rinse the dish with 2 mL of NRI medium to recover all fragments.
- Spin down the rosette fragments at 112 x g for 2 min.
- Aspirate the supernatant.
- Gently resuspend the pellet in 1 mL of 1x DPBS (without calcium and magnesium) and gently pipette the rosette fragments up and down using a 1,000-µL pipette to partially dissociate them.
- Add 4 mL of complete NRI medium and count the cells using trypan blue and an automated cell counter (see the Table of Materials)
NOTE: Dilute 20 µL of cell suspension in 20 µL of Trypan blue. This step may be omitted if the cells cannot be brought into a single-cell suspension. If the rosette fragments do not look completely dissociated, rosette fragments derived from about 50 EBs/60-mm dish can be resuspended in 50 mL of complete NRI medium and plated, as indicated in Table 1. - Aspirate the standard matrix (or laminin) coating solution from the Petri dishes, plates, and/or MEA chips (from step 1.3.7). Do not let them dry.
- Plate the cells in a complete NRI medium according to the study plan (about 15,000 cells/cm2; see Table 1 for plating volume indications).
- Incubate the plates overnight at 37 °C and 5% CO2.
- On Day 10, perform a total medium change using a complete neuronal differentiation medium (ND); see the Table of Materials.
- Refresh the complete ND medium twice a week until Day 28.
Table 1.
Note on dissociated rosette plating density: if rosette fragments do not look completely dissociated, in order to reach a cell plating density of about 15.000 cells/cm2, dissociated rosette fragments deriving from about 50 EBs/1 x 60mm-dish can be resuspended in 50 mL of complete NRI medium and plated as follows (depending on the plate format):
| Multiwell plates / MEA | Growth area (cm2/well) | Volume of the cell suspension to plate per well (or MEA chip) | Maximum number of plates that can be plated (with 50 ml of cell suspension) |
| 96 wells | 0.3 | 100 ul | 5 |
| 48 wells | 0.7 | 220 ul | 4 |
| 24 wells | 2 | 625 ul | 3 |
| 12 wells | 4 | 1.25 ml | 3 |
| 6 wells | 10 | 3.125 ml | 2 |
| Single well MEA chip | 3.5 | 1.1 ml | 45 |
Representative Results
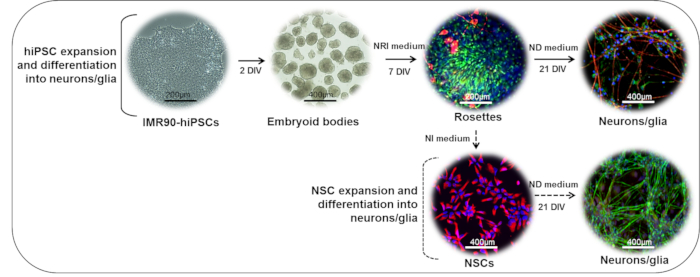
Figure 1: Schematic Representation of the Neuronal Differentiation Protocol. (Upper part) IMR90-hiPSC colonies can be cut into fragments to form embryoid bodies (EBs). After 2 days in vitro (DIV), EBs can be plated onto laminin- or standard matrix-coated dishes and cultured in the presence of neuroepithelial induction (NRI) medium to generate neuroectodermal derivatives (rosettes, here stained for nestin (green) and β-III-tubulin (red)). Rosettes can be dissociated, collected, replated on laminin- or standard matrix-coated dishes, and further differentiated into mature neuronal (NF200, red) and glial (GFAP, green) cells in the presence of neuronal differentiation (ND) medium. (Lower part) rosette-derived NSCs (nestin, red) can be expanded in the presence of neural induction (NI) medium, cryopreserved, or further differentiated in the presence of ND medium to form mixed neuronal (NF200, green) and glial (GFAP, red) cultures.
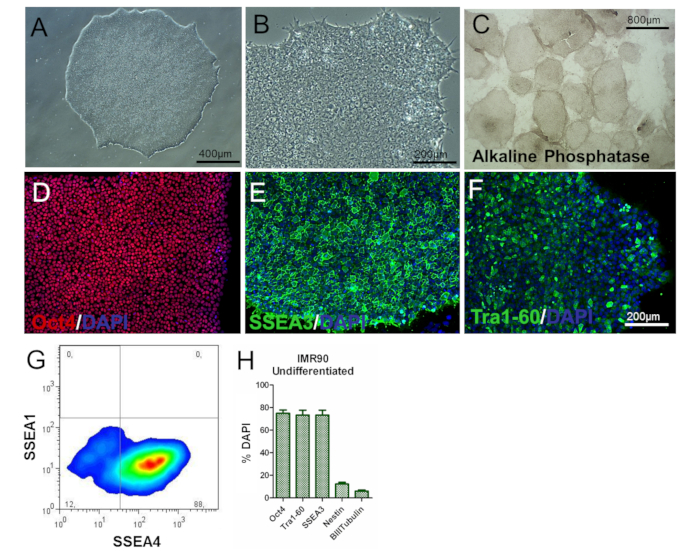
Figure 2. Characterization of Undifferentiated IMR90-hiPSCs. (A and B) Representative phase-contrast images (10X and 20X magnifications) of undifferentiated IMR90-hiPSC colonies. (C) representative images of alkaline phosphatase-stained colonies (4X magnification). (D-F) Representative immunocytochemical images of (D) Oct4 (red), (E) SSEA3 (green), and (F) Tra1-60 (green). (G) Representative dot plot of SSEA1 (CD15) and SSEA4 staining, analyzed by flow cytometry. (H) The bar graph shows the percentages of Oct4+ (~75 – 80%), Tra1-60+ (~75 – 80%), SSEA3+ (~75 – 80%), nestin+ (~10 – 15%), and β-III-tubulin+ (~3 – 7%) cells, counterstained with DAPI and quantified by HCI, with a mean of 3 to 5 biological replicates ± the S.E.M.
Divulgazioni
The authors have nothing to disclose.
Materials
| hiPSC EB medium: | |||
| Knockout DMEM | Thermo-Fisher | 10829-018 | (Step 2.1.7) |
| Knockout Serum Replacement (KOSR) | Thermo-Fisher | 10828-028 | 20% final concentration (Step 2.1.7) |
| Non-Essential Amino Acids | Thermo-Fisher | 11140-035 | (Step 2.1.7) |
| Penicillin/Streptomycin | Thermo-Fisher | 15140-122 | 50 U/mL final concentration (Step 2.1.7) |
| L-Glutamine 200 mM Solution | Thermo-Fisher | 25030-081 | 2 mM final concentration (Step 2.1.7) |
| β-Mercaptoethanol | Thermo-Fisher | 31350-010 | 50 µM final concentration (Step 2.1.7) |
| Complete neuroepithelial induction medium (NRI): | |||
| DMEM/F12 | Thermo-Fisher | 3133-038 | (Step 2.3.1) |
| Non-Essential Amino Acids | Thermo-Fisher | 11140-035 | (Step 2.3.1) |
| N2 Supplement | Thermo-Fisher | 17502-048 | (Step 2.3.1) |
| Penicillin/Streptomycin | Thermo-Fisher | 15140-122 | 50 U/mL final concentration (Step 2.3.1) |
| Heparin Grade I-A, ≥180 USP units/mg | Sigma-Aldrich | H3149-100KU | 2 µg/ml final concentration (Step 2.3.1) |
| bFGF | Thermo-Fisher | 13256-029 | 20 ng/ml final concentration added before use (Step 2.3.1) |
| Matrigel Basement Membrane Matrix | Corning | 354234 | 1:100 (Step 2.2). Thaw Matrigel on ice, prepare 200 ul aliquots and store them in -80°C. For coating, dilute 200 ul aliquot in 20 ml of cold DMEM/F12 medium. |
| Laminin | Sigma-Aldrich | L2020 | 1:100 (Step 2.2). Dilute in PBS 1X. |
| Complete Neuronal Differentiation medium (ND): | |||
| Neurobasal Medium | Thermo-Fisher | 21103049 | (Step 2.4.11) |
| B-27 Supplements (50x) | Thermo-Fisher | 17504044 | (Step 2.4.11) |
| N2 Supplement | Thermo-Fisher | 17502-048 | (Step 2.4.11) |
| Penicillin/Streptomycin | Thermo-Fisher | 15140-122 | 50 U/mL final concentration (Step 2.4.11) |
| GDNF | Thermo-Fisher | PHC7045 | 1 ng/ml final concentration. Added before use. (Step 2.4.11) |
| BDNF | Thermo-Fisher | PHC7074 | 2.5 ng/ml final concentration. Added before use. (Step 2.4.11) |

