Generating and Maintaining Astrospheres Using Human Pluripotent Stem Cells
Abstract
Source: Cvetkovic, C., et al. Synaptic Microcircuit Modeling with 3D Cocultures of Astrocytes and Neurons from Human Pluripotent Stem Cells. J. Vis. Exp. (2018).
The video demonstrates a method to generate astrospheres from progenitors from human pluripotent stem cells. It involves the growth and proliferation of stem cells with nutrient media containing growth factors and kinase inhibitors. These cells then aggregate and differentiate into astrocytes, forming astrospheres.
Protocol
1. Cell Culture and Reagent Preparation
- Prepare coated plates for cell culture.
- Dilute extracellular matrix (ECM) coating solution with DMEM/F12 media to prepare a 1 mg/mL stock solution. Aliquot the diluted ECM stock solution into 30 conical tubes of 3 mL each and immediately store in -20 °C. For a working solution, resuspend 3 mL of ECM stock into 33 mL of pre-chilled DMEM/F12 media, bringing the total volume to 36 mL for a concentration of 80 µg/mL.
- Coat 1 mL per well of a 6-well cell culture plate with ECM working solution. Add an additional 1 mL DMEM/F12 per well (if necessary) to ensure that the surface of the well is fully covered. Allow the coated 6-well cell culture plates to sit at room temperature for at least 1 h before use.
NOTE: Coated plates may be stored in the incubator or at 4 °C for up to 2 weeks.
- Prepare media formulations, growth factors, and small molecules.
- To prepare 500 mL of human pluripotent stem cell (hPSC) medium, add 20x and 500x supplements according to manufacturers' instructions.
- To prepare 500 mL of neural medium (NM), add heparin to a final concentration of 2 mg/mL, 1x of antibiotic/antimycotic solution, 1x B27 supplement or 1x N2 supplement to a 500 mL bottle of DMEM/F12 with L-glutamine supplement as previously detailed.
- To prepare a 10 mM (1,000x) stock solution of Rho-kinase inhibitor Y27632 (Y), add 10 mg of powder into 3 mL of phosphate-buffered saline (PBS). Filter, sterilize, aliquot, and store at -20 °C. Use at a 10 µM working concentration in media.
- To prepare a 20 mM (10,000x) stock solution of SB431542, add 10 mg of powder into 1.3 mL of dimethyl sulfoxide (DMSO). To prepare a 2 mM (1,000x) stock solution of DMH1, add 10 mg of powder into 13.1 mL of DMSO. Filter, sterilize, aliquot, and store each solution at -20 °C. Use each at a 2 µM working concentration in media (NM + SB431542 + DMH1).
NOTE: This protocol recommends 2 µM working concentrations of both SB431542 and DMH1 based on our observations and reports from others stating that this concentration effectively promotes neural induction from hPSCs. Higher concentrations have also been reported30. Additionally, with alternative media, it has also been shown that small molecules are not necessary for high neural conversion. Thus, working concentrations will vary depending on alternative cell culture environments, and optimal concentrations should be tested by individual researchers. - To prepare individual 100 µg/mL (10,000x) stock solutions of epidermal growth factor (EGF) and fibroblast growth factor-2 (FGF2), add 1 mg of each into 10 mL of PBS + 0.1% BSA. Aliquot EGF and FGF2 stock solutions into 50 µL aliquots and store at -80 °C. Use each at a 10 ng/mL working concentration in media; e.g., add 5 µL EGF and 5 µL FGF2 to 50 mL of NM (NM + EGF + FGF2).
- To prepare a stock solution of doxycycline hydrochloride (Dox), first dissolve powder in DMSO to 100 mg/mL and store at -20 °C. Further, dilute it to make a 2 mg/mL (1,000x) stock solution in PBS and store at -20 °C. Use at 2 µg/mL working concentration in media; e.g., add 50 µL Dox to 50 mL of NM (NM + Dox).
NOTE: Do not re-freeze solutions after thawing.
2. Generation of Neural Subtypes from Human Induced Pluripotent Cells (hPSCs)
NOTE: All cell cultures should be maintained in an incubator with 5% CO2 at 37 °C. These cultures are maintained at room oxygen levels, though lower levels may be utilized.
- Generate and maintain astrocyte progenitors (hAstros) from hPSCs.
NOTE: This section provides a brief and simplified differentiation protocol. For a detailed description (with embryoid body formation, rosette selection, and regional patterning steps), refer to the previously described protocol (Figure 1A, dotted green box).- Seed clusters of hPSCs (Figure 1B) onto ECM-coated 6-well plates (see step 1.1) with 2 mL of hPSC medium + Y per well. Feed the stem cells every day until they are about 50% confluent and split as needed.
- To split hPSCs, add 1 mL of passaging reagent to wells and aspirate after 1 min. Incubate cells at room temperature for 5 min, add 1 mL of hPSC medium, and split aggregates 1:10 onto new ECM-coated wells in 2 mL of hPSC medium + Y per well.
- Maintain the stem cells until they are about 50% confluent, then change media to NM + SB431542 + DMH1 to induce neural differentiation (day 0). When cells are about 95% confluent, split 1:6 into new ECM-coated wells in the same medium.
- On day 14, dissociate the cells with the detachment solution and transfer them to a non-coated flask with Y to promote the formation of aggregates.
NOTE: The addition of Y is utilized to promote cell survival and sphere formation but is not included during every feed. - For the generation of neuronal progenitors and neurons (hNeurons), use these day-14 cells as described below. Alternatively, utilize these cells at later time points from monolayer or sphere cultures before neuronal maturation occurs or gliogenesis commences.
NOTE: By default, this protocol produces dorsal-cortical astrocytes. However, astrocyte subtypes can be regionally specified by the addition of patterning morphogens if desired. Retinoic acid (RA) may be added to caudalize cells into spinal cord phenotypes, while sonic hedgehog (SHH), smoothened agonist (SAG), or purmorphamine will produce ventral phenotypes. - For the generation of astrocyte progenitors and astrocytes in spontaneously-formed 3D aggregates (hAstrospheres), switch to NM + EGF + FGF2 and feed weekly (or as needed to ensure stable pH) as previously detailed.
- Gently dissociate hAstro aggregates with detachment solution when dark centers appear and remove spheres that spontaneously attach. Break spheres gently once a week to maintain sphere health and to avoid necrotic cores.
NOTE: Do not exceed 5 min of treatment with detachment solution. - After 4-6 months of expansion, confirm cell identity and either freeze in cryopreservation medium (according to manufacturer's instructions) or alternative storage conditions for long-term preservation in liquid nitrogen or use immediately for experimentation.
- Gently dissociate hAstro aggregates with detachment solution when dark centers appear and remove spheres that spontaneously attach. Break spheres gently once a week to maintain sphere health and to avoid necrotic cores.
- Seed clusters of hPSCs (Figure 1B) onto ECM-coated 6-well plates (see step 1.1) with 2 mL of hPSC medium + Y per well. Feed the stem cells every day until they are about 50% confluent and split as needed.
Representative Results
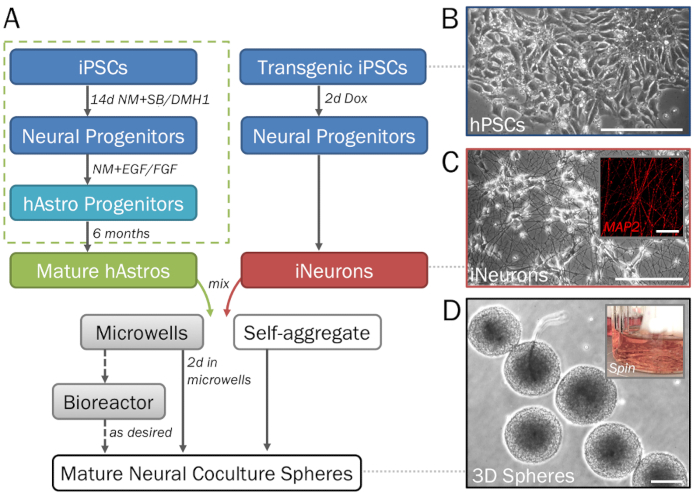
Figure 1: Stepwise depiction of the differentiation and formation of 3D neural spheres derived from hPSCs. (A) Timeline of key steps in the protocol. (B) Pure populations of neural cells can be generated from human induced pluripotent stem cells (hPSCs). For the generation of astrocyte progenitors (dotted green box), see step 2.1. (C) Inducible neurons (iNeurons; see section 2.2) generated from transgenic hPSCs via induced overexpression of neurogenin 2 demonstrate neuronal morphology on 2D ECM (day 7) and are positive for MAP2 (inset). (D) Spheres removed from microwell plates demonstrate consistent size for high-throughput screening. Spheres may be cultured in a spinner flask bioreactor if desired to prevent fusion. Scale bar = 50 µm (C, inset). Scale bars = 200 µm (A, D).
Divulgazioni
The authors have nothing to disclose.
Materials
| 6 well plate | Fisher Scientific | 08-772-1B | |
| 15 ml conical tubes | Olympus Plastics | 28-101 | |
| Accutase | Sigma | A6964-100ML | Detachment solution |
| AggreWell plate | Stemcell Technologies | 34850 | |
| Anti-Adherence Rinsing Solution | Stemcell Technologies | 7010 | Prevent cell adhesion to microwell plates |
| Anti/anti | Thermofisher | 15240062 | |
| B27 | Thermofisher | 17504044 | Media Supplement |
| BrainPhys neuronal medium | Stemcell Technologies | 5790 | Neurophysiological basal medium alternative |
| Cryostor CS10 | Stemcell Technologies | 7930 | Cryopreservation medium with 10% DMSO |
| DMEM/F12 | Thermofisher | 10565-042 | With GlutaMAX supplement |
| DMH-1 | Stemcell Technologies | 73634 | HAZARD: Toxic if swallowed. Working concentration: 2 uM |
| Doxycycline Hydrochloride (Dox) | Sigma | D3072-1ml | HAZARD: Toxic for pregnant women. Working concentration: 2 ug/mL |
| Epidermal growth factor (EGF) | Peprotech | AF-100-15 | Working concentration: 10 ng/mL |
| Fibroblast growth factor-2 (FGF) | Peprotech | 100-18B | Working concentration: 10 ng/mL |
| Hemacytometer or automatic cell counter | Life Technologies | AMQAX1000 | |
| Matrigel membrane matrix | Corning | 354230 | ECM coating solution. Working concentration: 80 ug/ml. Prepare on ice and ensure that pipettes, tubes, and media are pre-chilled. |
| Mounting solution | |||
| N2 | Thermofisher | 17502048 | Media Supplement |
| Phosphate buffered saline (PBS) | Stemcell Technologies | CA008-300 | |
| ReLeSR | Stemcell Technologies | 5872 | Detachment and passaging reagent |
| Rho-Kinase Inhibitor Y27632- (Y) | Tocris | 1254 | Working concentration: 10 uM |
| SB431542 | Stemcell Technologies | 72234 | Working concentration: 2 uM |
| T25 Culture Flask | Olympus Plastics | 25-207 | Vented caps |
| T75 Culture Flask | Olympus Plastics | 25-209 | Vented caps |

