Generating Rabbit Model of Bone Infection: A Technique of Inducing Chronic Infection by Injecting Bacteria Directly in Bone Marrow
Abstract
Source: Zhang, Y et al., Treatment with Vancomycin Loaded Calcium Sulphate and Autogenous Bone in an Improved Rabbit Model of Bone Infection. J. Vis. Exp. (2019).
This video describes the technique of surgically inducing bacterial infection in the bone marrow of a rabbit by using bone wax to avoid the leakage of bacteria. This rabbit model can be used to study bone infection treatment and bone regeneration.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of Bone Infection Models
- Keep male New Zealand white rabbits, aged 3 months, in individual cages, under air-controlled conditions (20 ± 1 °C) and 12 h/12 h light-dark illumination cycles. Offer routine diet and tap water daily.
- Ensure that at the time of surgery that the rabbit weighs more than 3 kg.
- Anaesthetize rabbits by intraperitoneal injection with pentobarbital sodium (3 mg per 100 g of body weight). Make sure rabbits are fully anesthetized by a failure to respond to a paw pinch. Fix rabbits on the operating table during operation procedure.
NOTE: Make sure that the modeling procedure duration is less than 1 h. - Shave the proximal tibia region using an electric shaver against the direction of hair growth. Disinfect the skin by applying a povidone-iodine solution.
- Mark the upper end of the tibia and the drilling hole position for injection with S. aureus (the distance to the upper end of the tibia is 1.5 cm) with pen and ruler. Make sure the drilling hole positions are in the middle of the tibial plateau horizontally (Figure 1A).
- Cut tibia skin using a No. 11 scalpel and make a 1 cm incision in the periosteum (Figure 1B, C). Punch a 2 mm diameter hole in the tibia using an electric bone drill unit (Figure 1D).
- Press the 2 mm diameter holes in the tibial plateau with a cylinder of bone wax of 2 mm diameter and 2 mm height (Figure 1E). Remove the spare bone wax along the horizontal plane of the tibial plateau (Figure 1F). Check that the 2 mm hole is full of bone wax (Figure 1G).
NOTE: Ensure that the holes are full of bone wax by checking the hole with or without blood overflow. - Sew up the periosteum and skin with absorbable surgical suture in a vertical mattress suture to prevent the animal from chewing the sutures (Figure 1H).
- Inject 1 x 108 CFU/mL of S. aureus solutions (30 µL per 100 g of body weight) with a 1 mL asepsis injector (Figure 1I). Make sure the needles penetrate the bone wax and inject the S. aureus solution into bone marrow slowly.
- Keep the animal in warm and clean conditions to avoid heat loss after modelling. Monitor respiratory rate and heart rate. After waking up, house the rabbits in individual cages with free access to food and water.
Representative Results
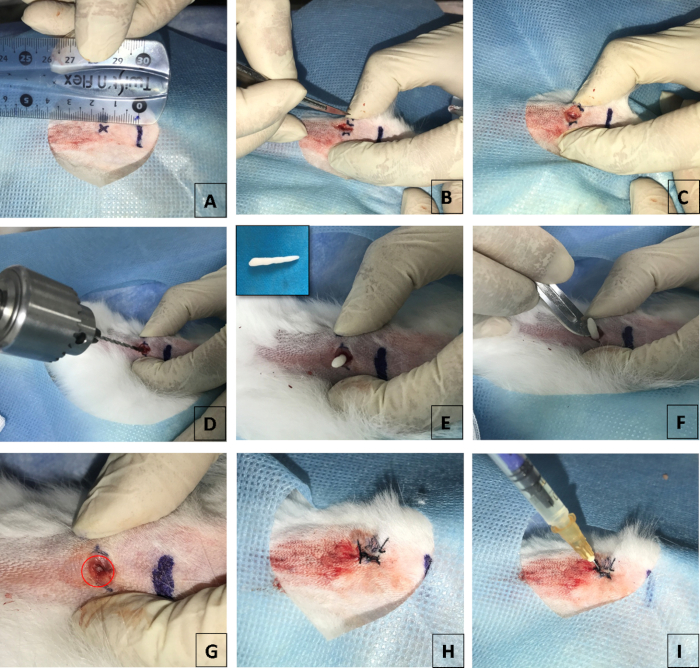
Figure 1. Surgical preparation of the bone infection model. (A) Shows the punching position on the tibia. The distance between the drilling hole position for injection with S. aureus to the upper end of tibia is 1.5 cm. (B) Incision made in the skin to expose the periosteum. (C) Shows incision made through the periosteum to expose the tibia. (D) Punch a 2-mm diameter hole in the tibia. (E) Fill the 2 mm diameter hole full of bone wax. (F) Cut off spare bone wax. (G) Show the bone wax filling the bone defect. (H) Sew up the periosteum and skin. (I) Inject with S. aureus solution.
Divulgazioni
The authors have nothing to disclose.
Materials
| absorbable surgical suture | Jinghuan | 18S0604A | |
| asepsis injector | Jinglong | 20170501 | |
| bone wax | ETHICON | JH5CQLM | |
| Electric bone drill unit | Bao Kang | BKZ-1 | |
| Electric shaver | Codos | 3800 | |
| New Zealand white rabbits | Zhejiang Experimental Animal Center | SCXK 2014-0047 | |
| No. 11 scalpel | Yuanlikang | 20170604 | |
| pentobarbital sodium | Merk | 2070124 | |
| S. aureus freeze drying powder | China General Microbiological Culture Collection Center | ATCC 6538 |

