Culturing and Harvesting of HSPC Clones: A Method to Obtain the Singly-Sorted HSPC Clones
Abstract
Source: Huber, A. R., et al. Characterizing Mutational Load and Clonal Composition of Human Blood. J. Vis. Exp. (2019).
This video describes the protocol for isolating HSPCs from the bone marrow and sorting them using a fluorescent activated cell sorting technique, which is a method to obtain singly-sorted HSPCs. We culture these sorted cells to obtain HSPC clones.
Protocol
1. HSPC Isolation, Sorting, and Culture
- Spin down at 1–2 x 107 mononuclear cells for 5 min at 350 x g and resuspend in 50 μL of FACS buffer. Resuspend the cell pellet in 100 μL of anti-CD3 staining solution (1:100 dilution of anti-CD antibody in FACS buffer). Transfer the cells to a microcentrifuge tube.
NOTE: When sorting with >2 x 107 cells, increase the antibody mix and FACS buffer volumes accordingly. - Prepare 50 μL of 2x HSC staining mix according to the recipe seen in Table 1
- Mix 50 μL of cell solution with the prepared HSC staining mix and incubate the cells for 15 min at room temperature (RT) or for 1 h on ice for the antibodies to bind.
- Wash the cells by adding 1 mL of FACS buffer and pellet by centrifuging for 5 min at 350 x g.
- Resuspend the cells in 300 μL of FACS buffer and filter the cell suspension through a 35 µm cell strainer-capped 5 mL polystyrene tube to remove cell clumps before fluorescence-activated cell sorting (FACS).
- Prepare 25 mL of HSPC culture medium, consisting of 1x SFEM medium supplemented with 100 ng/mL SCF, 100 ng/mL Flt3, 50 ng/mL TPO, 10 ng/mL IL-3, 20 ng/mL IL- 6, and 100 ng/mL antibiotic formulation (see Table of Materials).
- Fill a 384 well cell culture plate with 75 μL of HSPC culture medium in each well.
NOTE: To prevent evaporation of the medium in the outer wells, fill the outer wells with 75 μL of sterile water or PBS, and do not use these wells for cell sorting. - Sorting single HSPCs
- Set gates for the HSPC sorting based on an unstained control (singly-sorted HSCs) and 10,000 cells from the stained sample. A representative result for setting gates is depicted in Figure 1. Gate single cells by drawing a gate around the linear FSC-height vs. FSC-area fraction. Use unstained control fraction to draw gate for lineage– fraction. Draw gates for CD34+ cells and further characterize this subset by setting a specific gate for CD38– CD45RA– cells.
- Load the 384 well plate on the FACS machine and sort single cells.
NOTE: If applicable to the FACS-machine, toggle on the option to keep index sorting data to enable re-tracing of the sorted cells.
- Culturing singly-sorted HSCs
- Directly transfer the 384 well plate to a humidified 37 °C incubator with 5% CO2.
NOTE: To prevent evaporation during culture, wrap the 384 well culture plate (with lid) in transparent polyethylene wrap. - Keep the 384 well plate in the incubator for 3–4 weeks until visible clones appear. Representative images of clonal culture are depicted in Figure 2. Based on the condition of the input material 5%–30% of sorted cells will clonally expand.
- Directly transfer the 384 well plate to a humidified 37 °C incubator with 5% CO2.
| Antibody | volume [μL] |
| BV421-CD34 | 5 |
| FITC-Lineage mix (CD3/14/19/20/56) | 5 |
| PE-CD38 | 2 |
| APC- CD90 | 0.5 |
| PerCP/Cy5.5 – CD45RA | 5 |
| PE/Cy7- CD49f | 1 |
| FITC -CD16 | 1 |
| FITC-CD11 | 5 |
| FACS Buffer | 25.5 |
Table 1: HSC sorting mix. Shown is a table indicating the dilutions of antibodies used to sort the HSCs.
2. Harvesting HSPC Clones
- After 4 weeks of culturing, determine which wells have a confluency of 30% or higher.
- Pre-fill (for each clonal outgrowth) 1.5 mL microtubes with 1 mL of 1% BSA in PBS and label the tube according to the corresponding well.
- Pre-wet a pipette tip with 1% BSA in PBS to minimize the number of cells sticking to the pipette tip.
- Pipet up/down the medium in the well fiercely (at least 5 times) with a 200 μL pipette (set at 75 μL) and scrape the bottom of the well to loosen cells in the well, and collect the cell suspension in the labeled microtube corresponding to the well.
- Take up 75 μL of fresh 1% BSA in PBS and repeat pipetting in the well to ensure maximum uptake of cells.
NOTE: Clonally cultured cells can stick to the bottom of well. Inspect the wells using a standard inverted light microscope to ensure whether all cells have been collected. - If all wells with >30% confluency have been harvested, place the 384 well plate back in incubator. Clonal cultures can proliferate for up to 5 weeks.
- Spin down the cell suspension for 5 min at 350 x g. A small pellet should be visible.
- Carefully remove all but about 5 μL of supernatant. Cell pellets can be frozen at -20 °C and stored for multiple months before DNA isolation.
Representative Results
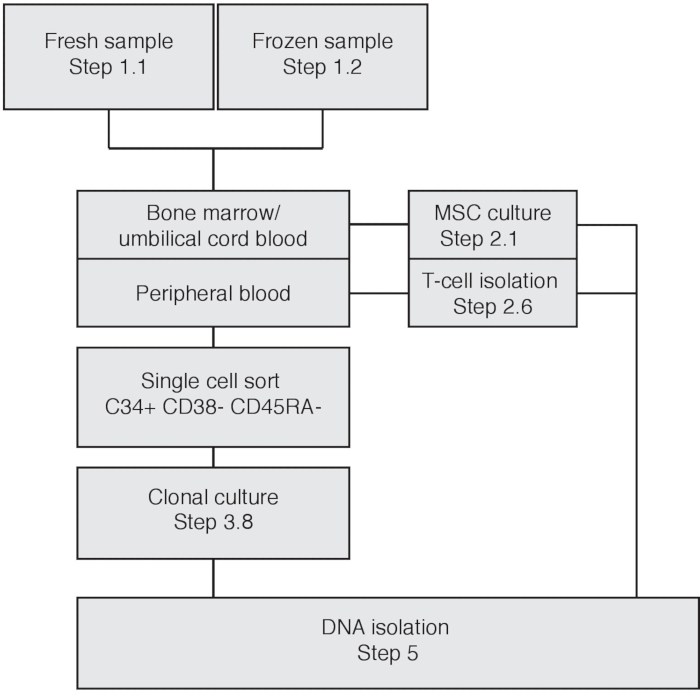
Figure 1: Flowchart depicting experimental procedure based on input material. Please click here to view a larger version of this figure.
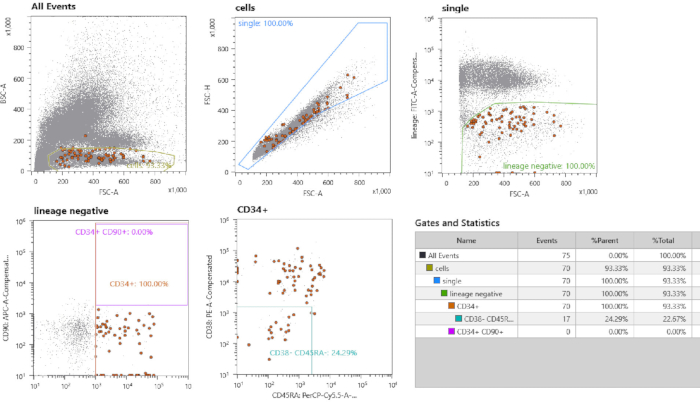
Figure 2: Cell sorting strategy. First, gating is performed on small mononuclear cells. Second, single cells are gated by selection of the linear fraction. Lineage negative cells are gated. All CD34+ CD38– CD45– cells are single cell-sorted. The fraction of cells in brown should be noted, which are the sorted cells highlighted by the option “index sorting”. Please click here to view a larger version of this figure.
Divulgazioni
The authors have nothing to disclose.
Materials
| CD11c FITC | BioLegend | 301603 | Clone 3.9 |
| CD16 FITC | BioLegend | 302005 | Clone 3G8 |
| CD3 BV650 | BioLegend | 300467 | Clone UCHT1 |
| CD34 BV421 | BioLegend | 343609 | 561 |
| CD38 PE | BioLegend | 303505 | Clone HIT2 |
| CD45RA PerCP/Cy5.5 | BioLegend | 304121 | Clone HI100 |
| CD49f PE/Cy7 | BioLegend | 313621 | Clone GoH3 |
| CD90 APC | BioLegend | 328113 | Clone 5E10 |
| Cell Strainer 5 mL tube | Corning | 352235 | |
| CELLSTAR plate, 384w, 130 µL, F-bottom, TC, cover | Greiner | 781182 | |
| Human Flt3-Ligand, premium grade | Miltenyi Biotech | 130-096-479 | Reconsititute in single-use aliquots (25 μL) at 100 μg/mL in 0.1% BSA in PBS |
| Human Recombinant IL-3 (E. coli-expressed) | Stem Cell Technologies | 78040.1 | Reconsititute in single-use aliquots (2.5 μL) at 100 μg/mL in 0.1% BSA in PBS |
| Human Recombinant IL-6 (E. coliexpressed) | Stem Cell Technologies | 78050.1 | Reconsititute in single-use aliquots (5 μL) at 100 μg/mL in 0.1% BSA in PBS |
| Human SCF, premium grade | Miltenyi Biotech | 130-096-695 | Reconsititute in single-use aliquots (25 μL) at 100 μg/mL in 0.1% BSA in PBS |
| Human TPO, premium grade | Miltenyi Biotech | 130-095-752 | Reconsititute in single-use aliquots (12.5 μL) at 100 μg/mL in 0.1% BSA in PBS |
| Lineage (CD3/14/19/20/56) FITC | BioLegend | 348701 | Clones: UCHT1, HCD14, HIB19, 2H7, HCD56 |
| PBS | Made in at Institute's facility. Commerically available PBS can also be used | ||
| Primocin | Invivogen | ant-pm-1 | Antibiotic formulation |

