Measuring the 50% Haemolytic Complement (CH50) Activity of Serum
Summary
The classical pathway is activated by antibody and culminates in target cell lysis. The CH50 assay provides a measure of the complement activity of a serum sample. This video demonstrates the steps involved in determining the CH50 of a serum sample, the calculations and interpreting of results.
Abstract
Protocol
Preparation of 5x Veronal Buffered Saline (VBS)
- To prepare the Veronal Buffered Saline (VBS), three separate solutions need to be prepared.
- Prepare solution 1 by dissolving 21.25gm of NaCl and 0.94gm of Sodium Barbitone in 350ml of distilled water. The final concentrations of NaCl and Sodium Barbitone are 1.02M and 13mM respectively.
- Prepare solution 2 by dissolving 1.44gm of Barbitone in 125ml of hot distilled water. The final concentration of Barbitone is 62.5mM.
- Prepare solution 3 by dissolving 20.33gm of MgCl2 and 4.41gm of CaCl2 in 100ml of distilled water. The final concentration of MgCl2 and CaCl2 is 2.18M and 440mM respectively.
- Mix solutions 1 and 2 and cool to room temperature.
- Once the combined solution has cooled, add 1.25ml of solution 3 and adjust the pH to 7.3-7.5 using 1M HCl.
- Adjust the final volume to 500ml with distilled water to prepare a 5x stock solution.
- To prepare a 1x working solution, dilute the stock 1:5 with distilled water.
Sensitisation of sheep red blood cells with haemolysin
- Prepare the haemolysin by firstly diluting it 1:50 with VBS
- To 4ml of SRBC add 6ml of VBS and gently mix by inversion
- Centrifuge at 600g x 5minutes
- Discard the supernatant and wash the cells another 2 times
- After the final wash, centrifuge the cells at 900g x 5minutes to pack the cells
- Discard the supernatant and resuspend the cells in sufficient VBS to prepare a 10% solution i.e. 0.5ml of packed cells are resuspended in 5 ml of buffer
- Dropwise add an equal volume of haemolysin (rabbit anti-sheep red blood cell antibody) to the cells while swirling continuously
- Incubate at 30°C for 30 minutes in a water bath
- Gently mix the cells every 15minutes
- Sensitised SRBC can be stored overnight at 4°C
CH50 assay
- Label a series of tubes in duplicate with 1:8, 1:16, 1:32, 1:64 and 1:128.
- Prepare a series of two fold serial dilutions of control and test serum in VBS each in duplicate
- Start at 1:4 (100ml serum + 300ml VBS) and transfer 200ml of sample into the next labelled tube.
- Mix thoroughly between dilutions and transfer 200ml to the next dilution with a fresh pipette tip.
- Repeat until all five dilutions are made.
- Discard 200ml from the final 1:128 dilution.
- Add 200ml of suspended sensitised SRBC to all tubes.
- Label two separate tubes as BLANK and add 200ml of sensitised SRBC + 200ml VBS. These tubes will measure spontaneous lysis of the SRBC in the VBS.
- Label another two separate tubes as TOTAL LYSIS and add 200ml of sensitised SRBC + 200ml distilled water.
- Gently mix all tubes.
- Incubate at 37°C for 30 minutes in a waterbath mixing after 15 minutes.
- Centrifuge the samples at 1,500g for 5 minutes to sediment the RBCs.
- Transfer 100ml of supernatant from each tube to a well in a 96 well flat bottom plate.
- Add 100ml of distilled water to each well.
- Read the absorbance of the samples at 540nm using a plate spectrophotometer.
Calculations
- Calculate the mean absorbance for each sample
- Subtract the BLANK absorbance (spontaneous lysis) from all samples
- Calculate the % lysis for each dilution using the following formula:

- Plot the percentage lysis (vertical axis) versus the serum dilution on the horizontal axis.
- Calculate the dilution required for 50% haemolysis for the control and test serum (Fig 2).
Representative Results:
The video includes an example of representative results. In practice, a control serum is also run at the same time as the test serum and is treated in the same manner. Below (Table 1) is sample data from a tested serum sample. The data is manipulated according to the equation presented in 5.3.
| Sample | OD540 | Mean | ||
| Blank | 0.042, 0.044 | 0.043 | ||
| Total lysis | 0.183, 0.183 | 0.183 | ||
| Dilution | OD540 | Mean | Mean-Blank | % Lysis |
| 1:8 | 0.200, 0.219 | 0.210 | 0.168 | 116 |
| 1:16 | 0.179, 0.173 | 0.176 | 0.134 | 93 |
| 1:32 | 0.134, 0.110 | 0.122 | 0.08 | 55 |
| 1:64 | 0.053, 0.066 | 0.059 | 0.017 | 12 |
| 1:128 | 0.044, 0.045 | 0.045 | 0.003 | 2 |
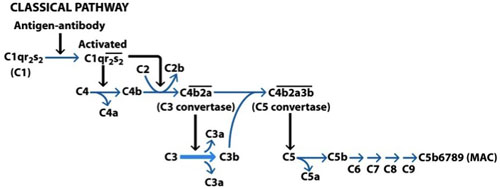
Figure 1. Activation of the classical complement pathway. The classical pathway is activated by binding immunoglobulin-M (IgM) or immunoglobulin-g (IgG) on the surface of a target cell. The Fc portion of the Ab binds to C1q, C1r is activated and this in turn activates another molecule of C1r which together activate two molecules of C1s. C1s now cleaves C4 which exposes the binding site for C2 which is also cleaved. The binding of C4b and C2a leads to the formation of a complex referred to as a C3 convertase. This complex now cleaves C3 forming C3a and C3b, some of which combine with the C3 convertase forming a C5 convertase. This complex now acts on C5, with the resulting C5b binding to C6 initiating the formation of the membrane attack complex (MAC). C5b6 acts on C7, which in turn act on C8 and ultimately on C9 resulting in the formation of the final MAC. (Goldsby et al, 2003).
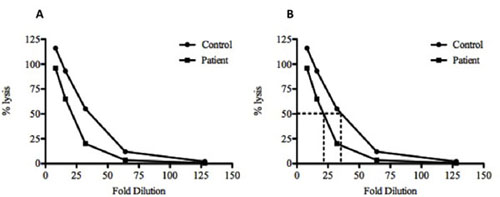
Figure 2. Plotting of sample data and calculating the CH50 for a control and test serum sample. (A), The calculated percentage (%) lysis of both the control (•) and test () serum samples are plotted against dilution factor. (B) To calculate the 50% lysis, a line is drawn from the 50% percent value until it intersects with the graph lines and then a vertical line is drawn down to the dilution. In this example, a 35-fold dilution of the control and 21.6-fold dilution of the test serum are required to achieve 50% lysis. This data indicates that there is less complement present in the test serum compared to the control as it required less dilution before 50% lysis was reached. The control sample also shows > 100% lysis at the 1:8 dilution. This is most likely due to incomplete lysis of the SRBC by the Distilled water and more efficient lysis by the complement components.
Discussion
The CH50 assay is subject to many interferences. Some SRBC are more fragile than others, resulting in spontaneous haemolysis that is unrelated to complement activity. The affinity of the rabbit antibody varies from lot to lot and from one manufacturer to another; this affects the amount of antibody that binds to the SRBC. Also, the process of sensitising SRBC with antibody results in cells with differing amounts of antibody coating the SRBC. Specimen collection and storage are an important potential source of error. Complement components e.g. C1q, C3, C4 and C5 are extremely labile, so proper sample handling is critical. Prolonged exposure to heat will decrease complement activity and will produce inactive fragments of complement components. To detect as many sources of error as possible, it is critical to test a control serum with a known CH50 value every time the assay is performed and to reproduce the accepted value of the known serum control. One way to determine if there are any differences between different batches of SRBC and haemolysin is to test new test materials against a standard sample of serum several times and then determine if there are any changes in the basal level of haemolysis or in the CH50 value for the control serum.
Acknowledgements
The author would like to thank Miss Lora Matthews for performing the technique and Mr Paul Shepherd for cinematography. This project was funded by the University of South Australia, Laboratory Medicine program.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Sheep red blood cells | CSL | 2490201 | Use at 1% final | |
| Serum | Human serum | |||
| Rabbit Anti-Sheep Haemolytic serum (RBCs), Unconjugated | AdB Serotec | C12HSB | ||
| 96 well flat bottom plate | Sarstedt | 83.1839 | ||
| Veronal buffered saline | Use at 1x final | |||
| Waterbath | ||||
| Plate reader | ||||
| 37°C room/incubator |

