3.3:
Molecular Models
33,682 Views
•
•
Physical models representing molecular architectures of chemical compounds play essential roles in understanding chemistry. The use of molecular models makes it easier to visualize the structures and shapes of atoms and molecules.
Skeletal Model
Simpler two-dimensional representations of chemical compounds are accomplished using skeletal models. The illustration shows only the molecular framework or bonds without explicitly showing the atoms. In this representation, many of the carbon atoms and hydrogen atoms are not explicitly shown. However, the positions of atoms are implied by the junctions or ends of the bonds. This model helps to represent larger and more complex chemical structures.
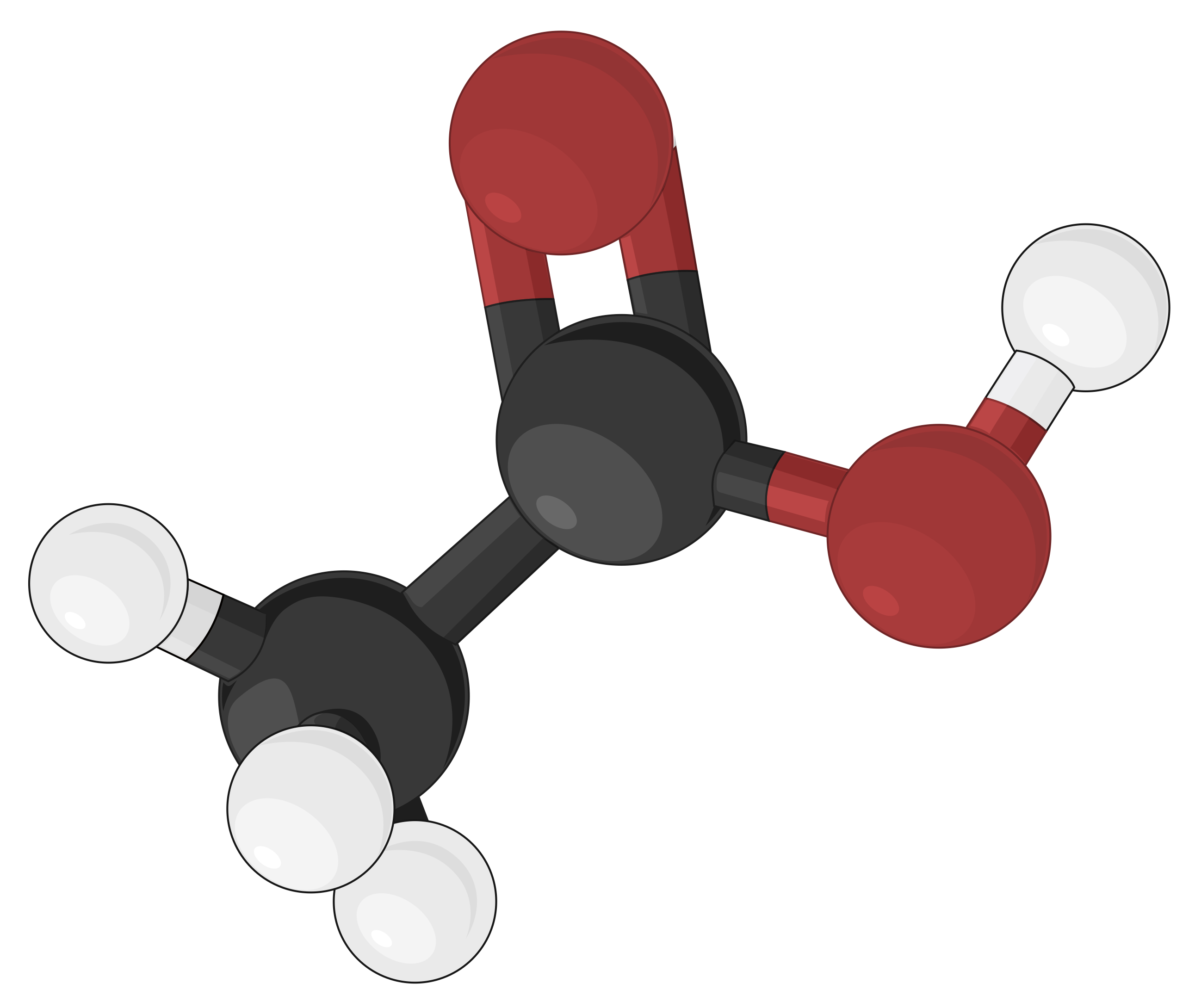
Ball-and-stick Model
Ball-and-stick models are three-dimensional models, where the atoms are depicted as color-coded balls or spheres, specific to different elements. The chemical bonds that connect the atoms are represented by rods and are easier to visualize. In doing so, the sizes of the balls are made relatively smaller, thereby compromising on the proportional correlation with the actual atomic size. Yet, the ball-and-stick model defines the angles between atoms, clearly depicting the molecular geometry of simple to more complex structures as compared to other molecular models.
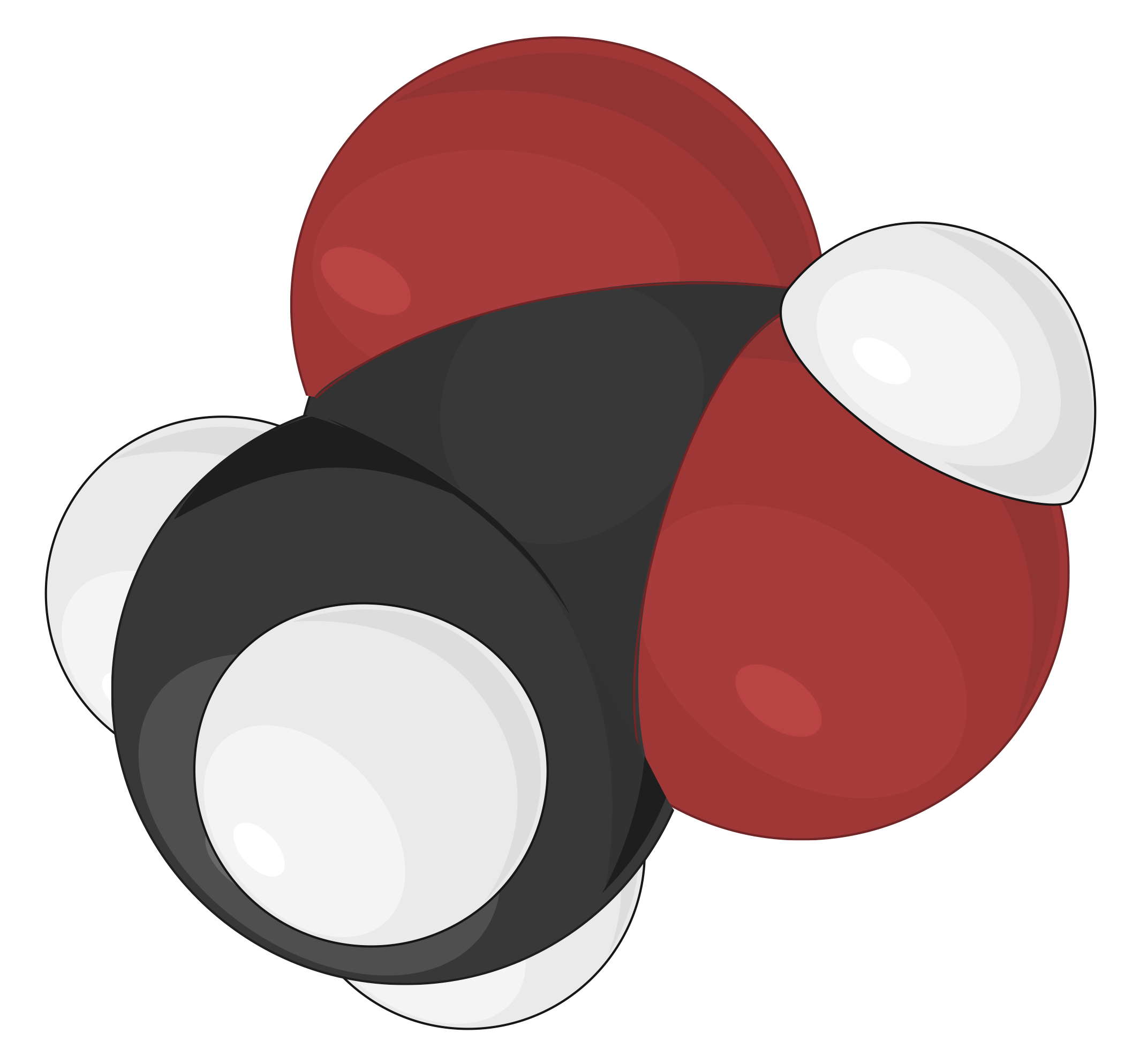
Space-filling Model
Space-filling models are most realistic, where the atoms are scaled up in size to fill the space between each other. The size and position of an atom in this model are determined by its bonding properties and van der Waals radius, or contact distance. The van der Waals radius describes how closely two atoms can approach each other when a covalent bond does not link them. The spheres in this model illustrate the relative space occupied by each atom within a compound, while the angles between atoms are not clearly visible
First designed by chemists Robert Corey and Linus Pauling, and later improved by Walter Koltun, the CPK coloring convention designates specific colors to atoms of each element. For example, according to the CPK convention, all hydrogen atoms are colored white, carbon atoms are black, nitrogen atoms are blue, oxygen atoms are red, sulfur atoms are deep yellow, and phosphorus atoms are purple. Alkaline earth metals are represented by dark green, and alkali metals are indicated by violet.
As an example, different molecular models of acetic acid (CH3COOH) can be represented in the following ways:
 |
 |
|
| Skeletal model | Ball-and-stick model | Space-filling model |
This text is adpted from: Openstax, Chemistry 2e, Section 2.4: Chemical Formulas.
