12.12:
Electrolytes: van’t Hoff Factor
29,448 Views
•
•
Colligative Properties of Electrolytes
The colligative properties of a solution depend only on the number, not on the identity, of solute species dissolved. The concentration terms in the equations for various colligative properties (freezing point depression, boiling point elevation, osmotic pressure) pertain to all solute species present in the solution. Nonelectrolytes dissolve physically without dissociation or any other accompanying process. Each molecule that dissolves yields one dissolved solute molecule. The dissolution of an electrolyte, however, is not this simple, as illustrated by the two common examples below:
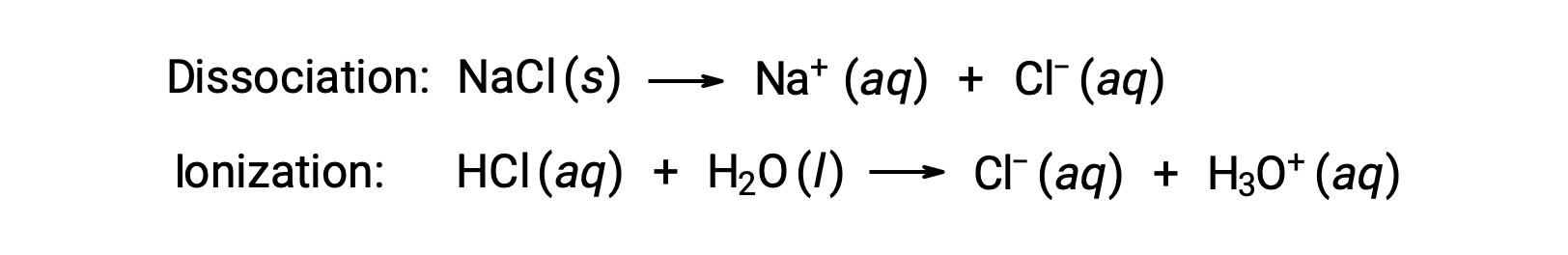
Considering the first of these examples, and assuming complete dissociation, a 1.0 m aqueous solution of NaCl contains 2.0 moles of ions (1.0 mol Na+ and 1.0 mol Cl−) per each kilogram of water, and its freezing point depression is expected to be
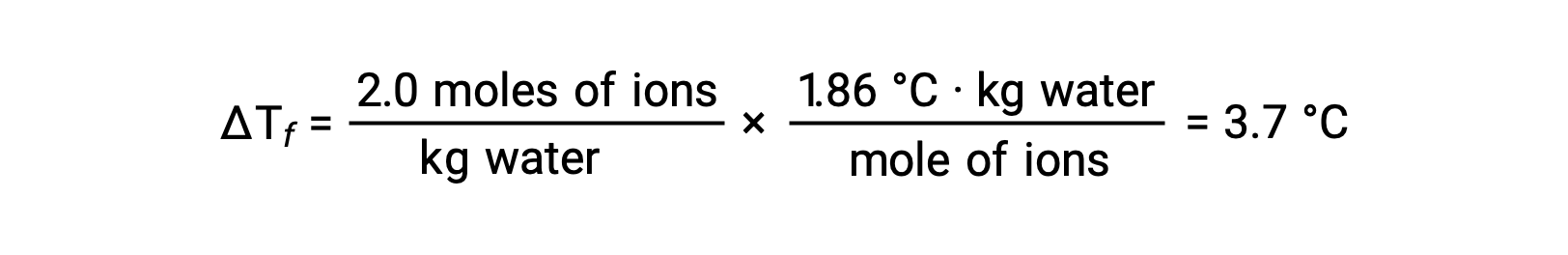
However, when this solution is actually prepared and its freezing point depression is measured, a value of 3.4 °C is obtained. Similar discrepancies are observed for other ionic compounds, and the differences between the measured and expected colligative property values typically become more significant as solute concentrations increase. These observations suggest that the ions of sodium chloride (and other strong electrolytes) are not completely dissociated in solution.
To account for this and avoid the errors accompanying the assumption of total dissociation, an experimentally measured parameter named in honor of Nobel Prize-winning German chemist Jacobus Henricus van’t Hoff is used. The van’t Hoff factor (i) is defined as the ratio of solute particles in solution to the number of formula units dissolved:

In 1923, the chemists Peter Debye and Erich Hückel proposed a theory to explain the apparent incomplete ionization of strong electrolytes. They suggested that although interionic attraction in an aqueous solution is very greatly reduced by solvation of the ions and the insulating action of the polar solvent, it is not completely nullified. The residual attractions prevent the ions from behaving as totally independent particles. In some cases, a positive and negative ion may actually touch, giving a solvated unit called an ion pair. Thus, the activity—or the effective concentration—of any particular kind of ion is less than that indicated by the actual concentration. Ions become more and more widely separated as the solution becomes more dilute and the residual interionic attractions become less and less. Thus, in extremely dilute solutions, the effective concentrations of the ions (their activities) are essentially equal to the actual concentrations. For 0.05 m solutions, the value of i for NaCl is 1.9, as opposed to an ideal value of 2.
This text is adapted from Openstax, Chemistry 2e, Section 11.4: Colligative Properties.
