Dissection, Immunohistochemistry and Mounting of Larval and Adult Drosophila Brains for Optic Lobe Visualization
Summary
This protocol describes three steps to prepare larval and adult Drosophila optic lobes for imaging: 1) brain dissections, 2) immunohistochemistry and 3) mounting. Emphasis is placed on step 3, as distinct mounting orientations are required to visualize specific optic lobe structures.
Abstract
The Drosophila optic lobe, comprised of four neuropils: the lamina, medulla, lobula and lobula plate, is an excellent model system for exploring the developmental mechanisms that generate neural diversity and drive circuit assembly. Given its complex three-dimensional organization, analysis of the optic lobe requires that one understand how its adult neuropils and larval progenitors are positioned relative to each other and the central brain. Here, we describe a protocol for the dissection, immunostaining and mounting of larval and adult brains for optic lobe imaging. Special emphasis is placed on the relationship between mounting orientation and the spatial organization of the optic lobe. We describe three mounting strategies in the larva (anterior, posterior and lateral) and three in the adult (anterior, posterior and horizontal), each of which provide an ideal imaging angle for a distinct optic lobe structure.
Introduction
The Drosophila visual system, comprised of the compound eye and underlying optic lobe, has been an excellent model for the study of neural circuit development and function. In recent years, the optic lobe in particular has emerged as a powerful system in which to study neurodevelopmental processes such as neurogenesis and circuit wiring1,2,3,4,5,6,7,8. It is made up of four neuropils: the lamina, medulla, lobula and lobula plate (the latter two comprise the lobula complex)1,2,3,4,5,6. Photoreceptors from the eye, target neurons of the lamina and medulla, which process visual inputs and relay them to the neuropils of the lobula complex1,2,3,4,5,6. Projection neurons in the lobula complex subsequently send visual information to the higher order processing centers in the central brain1,5,9. The complex organization of the optic lobe, necessitated by a need to maintain retinotopy and to process different types of visual stimuli, makes it an attractive system for studying how sophisticated neural circuits are assembled. Notably, the medulla shares striking similarities in both its organization and development with the neuroretina, which has long been a model for vertebrate neural circuit development3,8.
Optic lobe development begins during embryogenesis, with the specification of ~35 ectodermal cells that form the optic placode2,4,5,6,7,8. After larval hatching, the optic placode is subdivided into two distinct primordia: 1) the outer proliferation center (OPC), which generates the neurons of the lamina and outer medulla and 2) the inner proliferation center (IPC), which generates neurons of the inner medulla and lobula complex4,5,6,10. In the late second-instar larva, the neuroepithelial cells of the OPC and IPC begin to transform into neuroblasts that subsequently generate neurons via intermediate ganglion mother cells4,5,11,12. Optic lobe neuroblasts are patterned by spatially and temporally-restricted transcription factors, which act together to generate neural diversity in their progeny11,12,13,14. In the pupa, the circuits of the optic lobe neuropils are assembled via the coordination of several processes, including programmed cell death11,15, neuronal migration12,16, axonal/dendritic targeting10,17, synapse formation18,19 and neuropil rotations10,17.
Here, we describe the methodology by which larval and adult brains are dissected, immunostained and mounted for imaging the optic lobe. Given its complex three-dimensional organization, analysis of the optic lobe requires that one understand how its adult neuropils and larval progenitors are positioned relative to each other and the central brain. Thus, we put special emphasis on how the orientation of mounting relates to the spatial organization of the optic lobe structures. We describe three mounting strategies for larval brains (anterior, posterior and lateral) and three for adult brains (anterior, posterior and horizontal), each of which provide an optimal angle for imaging a specific optic lobe progenitor population or neuropil.
Protocol
1. Preparing larval brains for confocal imaging
- Dissections
NOTE: Before starting the dissection, prepare the fix (4% formaldehyde in phosphate buffered saline (PBS)) and PBT (0.1-0.3% Triton in PBS) solutions. The fix solution should be placed on ice during the dissection. Although paraformaldehyde (PFA) fixative is used in this protocol, alternative fixing strategies (using PLP20 or PEM21) have been described for specific epitopes. For larval dissections, two pairs of forceps (Dumont #5 or #55s) are needed. See the Table of Materials for more details.- Start by filling each well of the dissecting dish with 400 µL of 1x PBS. Use a pair of forceps to gently pick wandering third-instar larvae crawling on the inside of the vial. Place larvae in the first well of the PBS-filled dish.
- Take one larva and transfer it to the middle well. Using the non-dominant hand, hold the body of the larva down at the base of the well.
- With the dominant hand, gently grasp the larval mouth hook and pull away from the body. The larval brain should be attached to the mouth hook and other accessory tissue and will come off as the tissue is pulled away.
- Remove accessory imaginal discs and transfer the larval brain to the third well of the dissection dish. The imaginal discs are epithelial tissues surrounding the larval brain, which generate adult structures such as the antennae, eyes, legs and wings20. If left on the tissue, the imaginal discs may obstruct proper immunostaining of the larval brain.
- To remove the imaginal discs, use a pair of forceps to firmly grip the brain via the ventral nerve cord. Using another pair of forceps, gently pluck away the discs.
- Remove the eye disc carefully, since it wraps over the surface of the brain lobes. To remove the eye disc, use a pair of forceps to hold the brain against the base of the dish. Instead of holding the brain via the ventral nerve cord, use the forceps to lightly grasp the brain lobe being careful to not squeeze the lobe. Using another pair of forceps, gently pull away the eye disc.
NOTE: The eye imaginal disc will eventually give rise to photoreceptors of the retina. For those interested in studying neural connectivity between the retina and optic lobe, the eye disc can be left on the brain. However, for those solely interested in optic lobe structures, it is recommended to remove the eye disc as it may hinder image quality due to its location on the surface of the brain.
- Repeat steps 1.1.2‒1.1.4 until a sufficient number of brains have been collected (or 30 min of dissection time have elapsed). Dissections should not go longer than 30 min to ensure the integrity of the tissue is not compromised.
- Once the dissection period is over, use a P200 pipette to carefully remove PBS from the third well. Ensure that a small volume of liquid remains in the well to keep the brains immersed.
NOTE: It is crucial to keep the brains immersed in liquid at all points of the protocol. If the tissue dries out, the quality of the stain may be compromised by unwanted autofluorescence in the confocal image. - Add 500 µL of the cold fix solution into the third well. Use a pair of forceps to stir the liquid gently, allowing the brains to swirl in the dish. Cover the dish with a glass slide and place it on ice for 30 min to allow for tissue fixation.
- Remove the fixation solution and wash the brains with 400 µL PBT, 5 times.
NOTE: Triton is a detergent in PBT that keeps the brains from sticking to each other or the dish, and prepares the tissue for optimal antibody penetration.
- Immunohistochemistry
NOTE: There are several primary antibodies available from the Developmental Studies Hybridoma Bank (DSHB) that can be used to label specific optic lobe structures or cell types. DE-Cadherin labels the IPC and OPC neuroepithelia, Bruchpilot (Brp) marks the developing neuropil and Dachshund labels lamina and lobula neurons (as well as a small subset of medulla neurons). Additionally, Elav can be used to label neurons, Prospero to mark ganglion mother cells and Repo to identify glia.- Prepare the primary antibody solution by adding antibodies to PBT upto a total volume of 100 µL. A blocking agent (10% normal goat serum) can be used to prevent non-specific binding between the primary antibody and tissue20,22,23. The antibodies used in this protocol specifically label brain tissue without the need for blocking agents.
- Remove the final post-fixation wash and add the primary antibody solution. Ensure that there is only a small amount of PBT in the well prior to adding the antibody solution. After adding the solution, gently stir the brains with forceps 5‒10 times. Cover with a glass slide, seal with laboratory film and incubate at 4 °C overnight.
- Alternatively, if a refrigerated orbital shaker is available, place the dish on the shaker to optimize the overnight incubation.
NOTE: The primary antibody incubation and all subsequent steps can alternatively be performed in a 1.5 mL microcentrifuge tube. The volume of primary and secondary incubations can remain 100 µL, but the wash steps should be performed with 800 µL or more of PBT.
- Alternatively, if a refrigerated orbital shaker is available, place the dish on the shaker to optimize the overnight incubation.
- After incubation, wash out the primary antibodies with PBT as in step 1.1.8 of the previous section.
NOTE: The primary antibody solution should be saved and stored at 4 °C as it can be reused for subsequent experiments. Many primary antibodies can be reused up to four times. - Add 400 µL of PBT to the brains for a final wash. Cover the dish with a slide, seal with laboratory film and place it on an orbital shaker at low speed (100 rpm) and room temperature (RT) for ~4 h.
NOTE: The protocol can be paused here. Brains can be left in wash for 2‒3 days at 4 °C. - Prepare the secondary antibody solution in a total volume of 100 µL.
NOTE: In this protocol, all secondary antibodies are used at a dilution of 1:500, without blocking agents. - Remove the PBT wash from the well and add the secondary antibody solution. Mix the tissue in the solution using a pair of forceps. Cover the dish with a slide and laboratory film. Place the dish on an orbital shaker at RT for a minimum incubation period of 2 h. Since secondary antibodies contain light-sensitive fluorophores, cover the dish with aluminum foil.
NOTE: The protocol can be paused here. Brains can be left in secondary antibody solution overnight at 4 °C. - Wash out the secondary antibodies as described in step 1.2.3. Add 400 µL of PBT to the brains for a final wash. Cover the dish with a slide, seal with laboratory film and foil. Place the brains on a shaker at RT for 4 h.
NOTE: The protocol can be paused here. Brains can be left in wash for 2‒3 days at 4 °C.
- Mounting
- Remove final PBT wash and replace with two drops of fluorescence-safe mounting media. Place a drop of the mounting media onto the center of a slide.
- Transfer brains from the well to the drop on the slide using forceps. To avoid damaging the optic lobes, the brains can be held by the ventral nerve cord while transferring to the slide.
- Mount the brains as per the mounting strategies that follow (Figure 1A).
- Anterior side up
NOTE: For those interested in visualizing the anterior medulla, lamina or lobula plug, this mounting orientation is ideal. The best way to distinguish the anterior versus posterior mounts is by examining the position of the ventral nerve cord relative to the brain lobes.- Use the anterior orientation, to observe the ventral nerve cord projecting out over the lobes (Figure 1B).
- Posterior side up
NOTE: This orientation is recommended for those interested in visualizing the posterior tips of the OPC (pOPC) or IPC 10,11.- Use the posterior orientation, to view the ventral nerve cord projecting out from under the brain lobes (Figure 1C).
- Lateral view
NOTE: A lateral mounting orientation is used to visualize the crescent of lamina, medulla or lobula plug neurons in both the dorsal-ventral and anterior-posterior axes in a single plane (Figure 1G).- For a lateral mount, split the brain lobes from each other to fall flat on their side, with the lamina and lobula plug facing up.
- Using two pairs of sharp forceps, split the ventral nerve cord in half, starting from where the cord attaches to the lobes. Note that the two lobes are already separate from one another, with the ventral nerve cord holding them intact. Thus, split the ventral nerve cord down from the cleavage point between the lobes, to separate them. Then flip each brain lobe and attached ventral nerve cord on their sides with the lateral surfaces facing up.
NOTE: For lateral view mount, it is recommended to use a tungsten needle with a fine tip to orient the brain lobe on its side. To clearly visualize the orientation of the lobes and the direction of the ventral nerve cord when mounting, the microscope lighting can be adjusted. If using a gooseneck LED light source, the goosenecks should be positioned parallel to the surface of the slide for clear visibility. Also, the lighting intensity can be increased or decreased to enhance contrast between the brain structures and provide clarity while mounting.
- Anterior side up
- Place a small drop of PBS on either side of the mounting media containing the brains. Place one cover slip onto each drop, and a final coverslip over the brains. The right and left edges of the top coverslip should rest on the two other coverslips (Figure 1A).
NOTE: Prior to placing a coverslip over the brains, a bridge must be built. Larval brains are approximately 200 µm thick, and thus, to keep the integrity of the tissue, a bridge is created that increases the distance between the cover slip and the surface of the slide. - Seal the edges of the bridge with nail polish to secure the mounted brains.
- Image the brains using confocal microscopy.
2. Preparing adult brains for confocal imaging
- Dissections
NOTE: Adult brain dissections are more challenging than larval brains and require careful handling. It is strongly recommended to use at least one pair of ultra-fine forceps (Dumont #55) for this part of the protocol.- Anesthetize flies with CO2, using either a needle or flypad, and place them on a laboratory wipe or paper towel over ice (to keep them anesthetized).
- Add 400 µL of PBS to all three wells of the glass dish. Use a pair of forceps to gently grab one adult fly by the wings and place it into the first well of the dish.
- Use a pair of forceps held in the non-dominant hand to hold the thorax of the fly against the base of the well. With the dominant hand, gently pull off the head from the rest of the body.
- Transfer the head into the second well. Often, the head will float in the PBS and can be difficult to handle. Use a pair of forceps to hold it down against the base of the well by the proboscis.
- Peel away a region of the cuticle between the eyes using both forceps. Since the brain sits right below the cuticle, be as gentle as possible to prevent damaging the underlying tissue. Continue to peel cuticle away one piece at a time, until the brain is exposed. Remove any accessory tissue (i.e., retina, trachea, air sacs) that may remain attached to the brain.
NOTE: Removal of the retina has been described in previous protocols22,24, but the lamina can also be separated from the rest of the optic lobe. This is useful for those wanting to study the medulla or lobula complex, since the lamina sits at the surface and may obstruct visualization of these other optic lobe neuropils when imaging. To remove the lamina, use a pair of sharp forceps to gently pull at the indentation between the lamina and medulla neuropils. The lamina will slowly begin to peel away from the medulla. Repeat this motion until the entire lamina is removed. During this process it is important to maintain a firm grip of the brain. Use another pair of forceps to hold the brain against the base of the dish by positioning it so that the top and bottom of the central brain fit perfectly between the tips of the forceps. A firm grip around the central brain will prevent unwanted damage to the optic lobe. - Transfer the clean brain to the third well. Repeat steps 2.1.2‒2.1.6 until a sufficient number of brains are obtained or a maximum dissection period of 30 min has elapsed.
- Remove the PBS in the third well and add 500 µL of fix solution. Ensure that the brains do not dry out here or during any wash steps as this will lead to background fluorescence in the confocal images. Cover the dish with a glass slide and allow the brains to incubate in fix for 20 min at RT.
- After fixation, wash the brains with 400 µL of PBT, 5 times.
NOTE: The protocol can be paused here. Brains can be covered with a glass slide and laboratory film and left in wash for 2‒3 days at 4 °C. For researchers new to the system, it may be easier to leave accessory tissue on the brains before and during fixation. This will allow researchers to maximize the number of brain samples obtained in a given dissection period. After the tissue has been fixed and washed, the brains can be carefully cleaned before adding primary antibody solution. Adult brains with accessory tissue tend to float, and therefore risk drying out. As a result, it is recommended to use a pair of forceps to carefully pool all of the brains into the center of the dish upon adding fix solution, and to clean the brains immediately after fixation.
- Immunohistochemistry
- Perform immunohistochemistry as described for larval brains in section 1.2.
- Useful primary antibodies from the DSHB include those that are mentioned in the protocol of larval immunohistochemistry in section 1.2. See the Table of Materials for a complete list of antibodies and corresponding dilutions.
NOTE: Extra care must be taken during the wash steps for adult brains, since they may float to the surface of the wash solution and risk drying out on the sides of the well when the solution is removed (which will lead to background fluorescence). A good practice is to hold the pipette in one hand to add or draw away liquid, and a pair of forceps in the other to keep the brains submerged.
- Mounting
- Prior to the mounting step, perform a final wash with PBS (instead of PBT). PBS causes the brains to become slightly sticky, which allows them to remain in the desired orientation during mounting.
- Remove the final wash and replace with three drops of fluorescence-safe mounting media. Place a drop of the mounting media onto the center of a microscope slide.
- Transfer brains from the well to the drop on the slide using forceps. To avoid damaging the optic lobes and prevent the tissue from drying out, carefully retrieve a brain via capillary action. Slowly close a pair of forceps around a brain until a small volume of liquid containing the brain is drawn up between the tips.
NOTE: When transferring the brain to the slide, be careful to not close the forceps as this will damage the brain. Alternatively, a P200 pipette can be used to transfer the brains. Cut off the end of the tip to widen the opening and pre-rinse with PBT to prevent the brains from sticking to the inside of the tip. - Mount the brains as per the following strategies (Figure 2A).
- Anterior side up
NOTE: To visualize lamina and medulla neurons, orient the brain with the anterior side up (Figure 2B). This can be achieved by examining the anatomy and curvature of the central antennal lobes.- With a pair of forceps, gently turn the brain on its side to examine both the anterior and posterior sides. Observe that on the anterior side, the curvature of the brain is pronounced and the antennal lobes protrude slightly outward from the center.
- Posterior side up
- Position the brains with the posterior (flat) side facing up and the antennal lobes down (Figure 2C).
NOTE: This will bring the lobula and lobula plate closer to the imaging surface and is ideal for those interested in visualizing lobula complex neurons.
- Position the brains with the posterior (flat) side facing up and the antennal lobes down (Figure 2C).
- Horizontal view
NOTE: To visualize all optic lobe neuropils simultaneously, use a horizontal mounting strategy (Figure 2G). In this orientation, neuronal cell bodies, axonal trajectories and dendritic arborizations can be visualized in the same plane.- Initially, orient the brain with the anterior side up. With a tungsten needle, gently tilt the brain 90° upwards so that it sits on its dorsal side with its antennal lobes facing outwards. Check that both the anterior and posterior sides of the brain are visible in this orientation.
- Anterior side up
- Instead of a cover slip bridge, use clay to elevate the cover slip from the slide. The use of clay allows for re-mounting of the brains after imaging (described in the discussion section).
- Place a small piece of clay on each corner of a cover slip. Ensure that each piece of clay is relatively the same thickness. The pieces of clay should be between 0.5–1 mm in thickness. Gently place the coverslip over the brains and apply slight pressure to the corners. The coverslip should immediately catch the liquid and form a seal. The slide is now ready for imaging.
NOTE: For long-term storage of the slide, it is recommended to seal the coverslip with nail polish and store at 4 °C away from light.
- Place a small piece of clay on each corner of a cover slip. Ensure that each piece of clay is relatively the same thickness. The pieces of clay should be between 0.5–1 mm in thickness. Gently place the coverslip over the brains and apply slight pressure to the corners. The coverslip should immediately catch the liquid and form a seal. The slide is now ready for imaging.
Representative Results
Confocal images of larval and adult optic lobes mounted in the orientations described in the protocol are presented in Figure 1 and Figure 2.
Figure 1 shows schematics and representative confocal slices of larval brains positioned in the anterior, posterior and lateral orientations. In the anterior mounting orientation, the OPC epithelium (DE-Cadherin), medulla neuroblasts (deadpan>βgal) and lamina neurons (Dachshund) appear at the surface as bands of cells that wrap around the brain (Figures 1B,D). The OPC is spatially patterned along its dorsal/ventral axis by the differential expression of transcription factors6,11,12. Vsx1 labels the central OPC (cOPC), Optix labels the main OPC (mOPC) and Rx is expressed in the posterior OPC (pOPC), which represents the tips of the crescent11,12,25. The anterior mount is ideal for the visualization of the cOPC, as this region of the crescent lies at the surface in this orientation (Figure 1D). Neurons of the lamina are also visible on the lateral side of the lobe in this orientation. Deeper into the brain along the z-axis, additional regions of the OPC, as well as other structures, become visible (Figure 1E,F). At a middle point in the z-stack, the mOPC epithelium, along with its respective neuroblasts and neurons, are visible (Figure 1E). Additionally, the proximal region of the IPC (p-IPC) and the lobula plug, which give rise to neurons of the lobula and lobula plate6,13,26, are visible in these intermediate slices. The deepest z-slices depict the other side of the brain, where the pOPC tips are located (Figure 1F). The superficial tip of the IPC (s-IPC) is also present in these deepest slices13.
The structures and cells located at the bottom of an anteriorly-mounted brain correspond to those that would appear on the surface of a posteriorly-mounted brain. Due to their proximity to the imaging objective, the optic lobe structures closest to the surface of the brain resolve better in confocal images compared to those located at the bottom. At the surface, there is minimal light scattering between the tissue and the objective. In deeper parts of the tissue, more light scattering leads to a weaker fluorescence signal. Thus, a posterior mounting strategy permits optimal imaging conditions to visualize the tips of the OPC or ventral-IPC (Figure 1F), whereas an anterior mounting strategy is better-suited for visualizing cells of the lamina or cOPC (Figure 1D). If the region of interest is the lobula plug or mOPC and its progeny, either mounting orientation is suitable, as these structures are located towards the middle of the brain (Figure 1E).
A laterally-mounted larval brain lobe (Figure 1G) can be used to visualize the medulla, lamina or lobula plug neuronal crescents in one focal plane. Different structures can be visualized at different depths along the z-axis. At the surface, the lamina crescent (Eya) is visible with the lobula plug crescent located between its arms (Figure 1H). The medulla neuronal crescent (Bsh, Eya and Svp) appears at a slightly deeper z-position (Figure 1I). In a single z-slice one can visualize the entirety of the neuronal crescent along both the dorsal-ventral and anterior-posterior axes. Thus, this orientation is suitable for a researcher interested in determining where their gene of interest is expressed with respect to the spatial axes of the OPC.
Figure 2 shows schematics and representative confocal slices of adult brains positioned in the anterior, posterior and horizontal orientations. The lamina has been removed in these images to better show the underlying medulla and lobula complex. The medulla, lobula and lobula plate are each comprised of a cortex, which contains neuronal cell bodies, and a neuropil, which is made up of axonal and dendritic arborizations. In the anterior orientation (Figure 2B), the medulla cortex and neuropil are located at the surface, whereas in the posterior orientation (Figure 2C), the lobula and lobula plate are the first structures imaged. Figure 2D‒F display representative images of an anteriorly-mounted adult optic lobe at three Z-positions. Since the medulla is located at the surface of the anterior mount (Figure 2D), the medulla cortex (Vsx1) is immediately visible. Cell bodies in the cortex project their arborizations into the neuropil (labeled by Bruchpilot), which can be visualized at an intermediate z-position (Figure 2E). The lobula also appears at this level, located perpendicular to the medulla. At the deepest z-position, the lobula plate is visible (Figure 2F). Thus, researchers interested in studying the neurons of the lobula complex should use a posterior-mount orientation, whereas those interested in the lamina and medulla should mount their brains in an anterior orientation.
A horizontally-mounted optic lobe is achieved when the brain is flipped 90° on to its side from an initial anterior position (Figure 2G). In this view, all of the neuropils and cortices of the optic lobe are visible within a single plane (Figure 2H,I). This mounting orientation is recommended for the visualization of retinotopic projections and the projections of neurons that target multiple optic lobe neuropils.
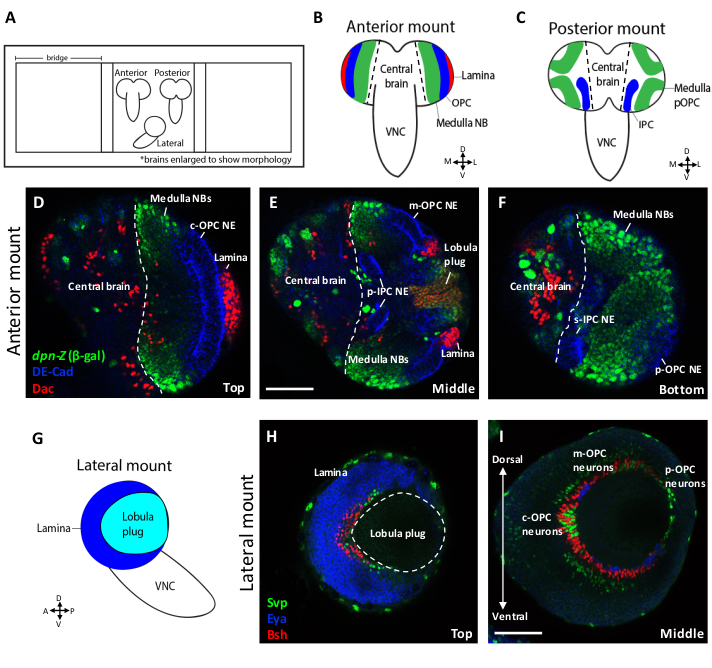
Figure 1. Larval brain mounting orientations. (A) Cartoon schematic showing late 3rd instar larval brains mounted on a slide in three orientations. Orientations can be distinguished from one another based on the position of the ventral nerve cord (VNC) relative to the brain lobes. (B) In the anterior mount, the VNC comes over the top of the brain lobes. In this orientation, the medulla neuroblasts (NBs), anterior OPC neuroepithelium (NE) and neurons of the lamina are visible. (C) In the posterior mount, the VNC protrudes from beneath the brain lobes. This orientation permits the visualization of both posterior (tip) medulla NBs and the ventral IPC NE. (D‒F) Confocal images of an anteriorly-mounted 3rd instar larval brain stained for the OPC and IPC NE marker DE-Cadherin (blue), lamina marker Dac (red) and NB marker dpn>lacZ (β-gal) (green). D-F are Z-slices from the top (D), middle (E) and bottom (F) of the same confocal stack. (G) Schematic depicting a late 3rd instar larval brain mounted in a lateral orientation. (H, I) Confocal images of a laterally-mounted 3rd instar larval optic lobe stained for the lamina marker Eya (blue), and lamina and medulla neuronal markers Bsh (red) and Svp (green). In this orientation, the lamina (H) and medulla (I) neuronal crescents can be visualized along the entire dorsal-ventral and anterior-posterior axes in a single plane. H and I are Z-slices from top (H) and middle (I) of the same confocal stack. Scale bars = 50 um. Please click here to view a larger version of this figure.
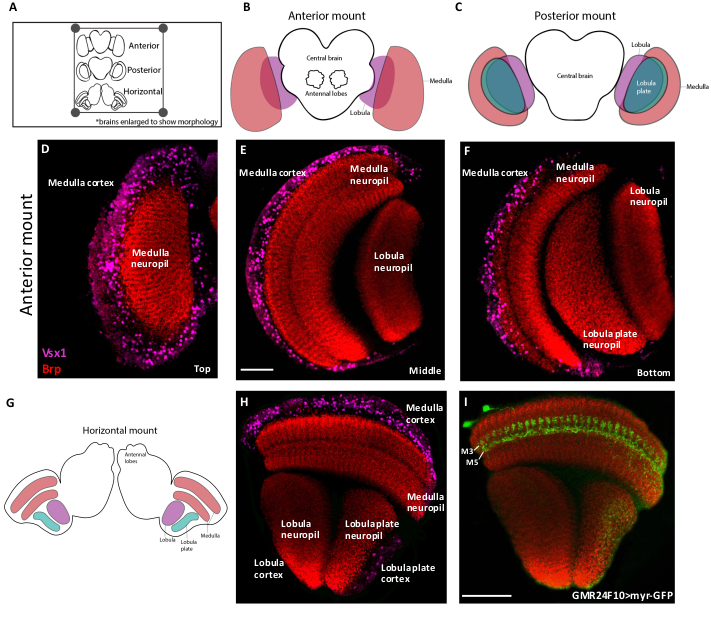
Figure 2. Adult brain mounting orientations. (A) Cartoon schematic showing adult brains mounted on a slide in three orientations. Orientations can be distinguished based on the location of the antennal lobes. (B) In the anterior orientation, the antennal lobes are facing up and the medulla neuropil is located at the surface. (C) In the posterior orientation, the brain is mounted with the antennal lobes facing down and the lobula and lobula plate neuropils located at the surface. (D‒F) Confocal Z-slices of an anteriorly-mounted adult brain labeled with the medulla neuronal marker Vsx1 (magenta) and the neuropil marker Brp (red). D-F are Z-slices from the top (D), middle (E) or bottom (F) of the same confocal stack. (G) Schematic of an adult brain in the horizontal orientation, which can be achieved by mounting the brain on its side. (H) Confocal image of a horizontally-mounted brain stained with Vsx1 (magenta) and Brp (red). In this mounting orientation all three optic lobe neuropils and cortices are visible in one Z-slice. This orientation is ideal for visualizing the morphologies of optic lobe neurons across the neuropils within a single plane. (I) Confocal image of a horizontally-mounted brain labeling a medulla neuron (Dm4) with GFP (green) and the neuropils with Brp (red). Dm4 neurons send arborizations to layers 3 and 5 of the medulla neuropil. Scale bars = 50 µm. Please click here to view a larger version of this figure.
Discussion
In this protocol, we describe a method to immunostain larval and adult Drosophila brains and mount them in several orientations. While methods to stain larval and adult brains have been previously described22,23,24,27,28, mounting strategies for the optimal visualization of specific optic lobe structures have received less attention28. It is anticipated that the protocol described here will provide researchers with a greater understanding of the relationship between mounting orientation and the optic lobe structures visualized.
In addition to the orientations described in this protocol, alternative angles of adult and larval optic lobe visualization can be achieved by separating the optic lobe from the central brain. The optic lobes can be split from the central brain using insect scissors, forceps or a tungsten needle. In the adult, this can be a useful strategy in cases where the curvature of the central brain inhibits flat mounting of the lobes, resulting in uneven angles during imaging. It should be noted that an isolated lobe will be more challenging to mount without the reference points provided by the central brain (i.e., antennal lobes, brain curvature, etc.) that are used to determine mounting orientation. This limitation can be overcome by analyzing optic lobes under a fluorescence GFP microscope (if the brain is stained for the appropriate fluorescent marker) to ensure the desired orientation is achieved before adding the coverslip. Similarly, in the larva, the removal of a brain lobe from the attached contralateral lobe and ventral nerve cord, allows the brain to be mounted in any orientation. A GFP-microscope can be used to determine the mounting angle with respect to the optic lobe structures of interest.
Brains can also be imaged in multiple orientations by removing the coverslip after imaging and remounting the brain. For remounting, the original bridge should be made with clay and nail polish should not be applied. To re-orient brains, a pair of forceps can be inserted underneath the coverslip to break the seal. Once the coverslip is lifted, most of the brains should remain in the mounting media. The brains can then be remounted and a new coverslip can be placed on top of the brains. This technique has previously been used to image a single brain in multiple orientations to build a high-resolution three-dimensional image of a medulla neuron’s morphology17. While remounting is often done with adult brains, the technique can also be applied to larval brains, which would also require building the bridge with clay. It is important to handle larval brain samples carefully when remounting because their fragility makes them more likely to tear when the coverslip is removed.
The above-mentioned protocols can also be applied to pupal brain tissue10,22,24. Since pupal brains undergo rapid morphogenic changes during development, the mounting orientations for early pupa (0‒30 h APF) resemble those of larval brains, whereas mid-late stage pupa (>50 h APF) are closer to adult brain mounting orientations. Pupal brains are more fragile than larval and adult brains, and therefore require extra care when being manipulated.
Finally, in addition to fixed and stained tissue, an understanding of brain mounting orientations is important for live imaging applications. Larval and adult brains can be cultured and imaged under live conditions to follow cell divisions and changes in neuronal morphology and activity over time24,27,29. Here, the mounting orientation used is critical, as the weaker endogenous fluorescence demands that cell types of interest are located as close as possible to the surface of the brain for optimal signal detection during imaging.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We would like to thank Claude Desplan for sharing with us an aliquot of the Bsh antibody. The DE-Cadherin, Dachshund, Eyes Absent, Seven-up and Bruchpilot monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. This work was supported by an NSERC Discovery Grant awarded to T.E.. U.A. is supported by an NSERC Alexander Graham Bell Canada Graduate Scholarship. P.V. is supported by an Ontario Graduate Scholarship.
Materials
| 10x PBS | Bioshop | PBS405 | |
| 37% formaldehyde | Bioshop | FOR201 | |
| Alexa Fluor 488 (goat) secondary | Invitrogen | A-11055 | use at 1:501 |
| Alexa Fluor 555 (mouse) secondary | Invitrogen | A-31570 | use at 1:500 |
| Alexa Fluor 647 (guinea pig) secondary | Invitrogen | A-21450 | use at 1:503 |
| Alexa Fluor 647 (rat) secondary | Invitrogen | A-21247 | use at 1:502 |
| Cover slips | VWR | 48366-067 | |
| Dissecting forceps – #5 | Dumont | 11251-10 | |
| Dissecting forceps – #55 | Dumont | 11295-51 | |
| Dissection Dish | Corning | 722085 | |
| Dry wipes | Kimbery Clark | 34155 | |
| Goat anti-Bgal primary antibody | Biogenesis | use at 1:1000 | |
| Guinea pig anti-Bsh primary antibody | Gift from Claude Desplan | use at 1:500 | |
| Guinea pig anti-Vsx1 primary antibody | Erclik et al. 2008 | use at 1:1000 | |
| Laboratory film | Parfilm | PM-996 | |
| Microcentrifuge tubes | Sarstedt | 72.706.600 | |
| Microscope slides | VWR | CA4823-180 | |
| Mouse anti-dac primary antibody | Developmental Studies Hybridoma Bank (DSHB) | mabdac2-3 | use at 1:20 |
| Mouse anti-eya primary antibody | DSHB | eya10H6 | use at 1:20 |
| Mouse anti-nc82 primary antibody | DSHB | nc82 | use at 1:50 |
| Mouse anti-svp primary antibody | DSHB | Seven-up 2D3 | use at 1:100 |
| Polymer Clay | Any type of clay can be used | ||
| Rabbit anti-GFP | Invitrogen | A-11122 | use at 1:1000 |
| Rat anti-DE-Cadherin primary antibody | DSHB | DCAD2 | use at 1:20 |
| Slowfade mounting medium | Invitrogen | S36967 | Vectashield mounting medium ( cat# H-1000) can also be used |
| Triton-x-100 | Bioshop | TRX506 |
Riferimenti
- Fischbach, K. -. F., Dittrich, A. P. M. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Research. 258, (1989).
- Hofbauer, A., Campos-Ortega, J. A. Proliferation pattern and early differentiation of the optic lobes in Drosophila melanogaster. Roux’s Archives of Developmental Biology. 198, 264-274 (1990).
- Sanes, J. R., Zipursky, S. L. Design Principles of Insect and Vertebrate Visual Systems. Neuron. 66, 15-36 (2010).
- Nériec, N., Desplan, C. From the Eye to the Brain. Development of the Drosophila Visual System. Current Topics in Developmental Biology. 116, 247-271 (2016).
- Apitz, H., Salecker, I. A Challenge of Numbers and Diversity: Neurogenesis in the Drosophila Optic Lobe. Journal of Neurogenetics. 28, 233-249 (2014).
- Contreras, E. G., Sierralta, J., Oliva, C. Novel strategies for the generation of neuronal diversity: Lessons from the fly visual system. Frontiers in Molecular Neuroscience. 12, 140 (2019).
- Erclik, T., Hartenstein, V., Lipshitz, H. D., McInnes, R. R. Conserved Role of the Vsx Genes Supports a Monophyletic Origin for Bilaterian Visual Systems. Current Biology. 18, 1278-1287 (2008).
- Erclik, T., Hartenstein, V., McInnes, R. R., Lipshitz, H. D. Eye evolution at high resolution: The neuron as a unit of homology. Biologia dello sviluppo. 332, 70-79 (2009).
- Wu, M., et al. Visual projection neurons in the Drosophila lobula link feature detection to distinct behavioral programs. eLife. 5, (2016).
- Ngo, K. T., Andrade, I., Hartenstein, V. Spatio-temporal pattern of neuronal differentiation in the Drosophila visual system: A user’s guide to the dynamic morphology of the developing optic lobe. Biologia dello sviluppo. 428, 1-24 (2017).
- Li, X., et al. Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature. 498, 456-462 (2013).
- Erclik, T., et al. Integration of temporal and spatial patterning generates neural diversity. Nature. 541, 365-370 (2017).
- Apitz, H., Salecker, I. A region-specific neurogenesis mode requires migratory progenitors in the Drosophila visual system. Nature Neuroscience. 18, 46-55 (2015).
- Suzuki, T., Kaido, M., Takayama, R., Sato, M. A temporal mechanism that produces neuronal diversity in the Drosophila visual center. Biologia dello sviluppo. 380, 12-24 (2013).
- Hara, Y., Sudo, T., Togane, Y., Akagawa, H., Tsujimura, H. Cell death in neural precursor cells and neurons before neurite formation prevents the emergence of abnormal neural structures in the Drosophila optic lobe. Biologia dello sviluppo. 436, 28-41 (2018).
- Morante, J., Erclik, T., Desplan, C. Cell migration in Drosophila optic lobe neurons is controlled by eyeless/Pax6. Development. 138, 687-693 (2011).
- Millard, S. S., Pecot, M. Y. Strategies for assembling columns and layers in the Drosophila visual system. Neural Development. 13, 1-17 (2018).
- Takemura, S. Y., Lu, Z., Meinertzhagen, I. A. Synaptic circuits of the Drosophila optic lobe: The input terminals to the medulla. Journal of Comparative Neurology. 509, 493-513 (2008).
- Akin, O., Bajar, B. T., Keles, M. F., Frye, M. A., Zipursky, S. L. Cell-type-Specific Patterned Stimulus-Independent Neuronal Activity in the Drosophila Visual System during Synapse Formation. Neuron. 101, 894-904 (2019).
- Spratford, C. M., Kumar, J. P. Dissection and immunostaining of imaginal discs from drosophila melanogaster. Journal of Visualized Experiments. (91), e51792 (2014).
- Dokucu, M. E., Zipursky, S. L., Cagan, R. L. Atonal, rough and the resoluton of proneural clusters in the developing Drosophila retina. Development. 122, 4139-4147 (1996).
- Hsiao, H. Y., et al. Dissection and immunohistochemistry of larval, pupal and adult Drosophila retinas. Journal of visualized experiments. , e4347 (2012).
- Kelly, S. M., Elchert, A., Kahl, M. Dissection and immunofluorescent staining of mushroom body and photoreceptor neurons in adult Drosophila melanogaster brains. Journal of Visualized Experiments. , e56174 (2017).
- Williamson, R. W., Hiesinger, R. P. Preparation of developing and adult Drosophila brains and retinae for live imaging. Journal of Visualized Experiments. , e1936 (2010).
- Bertet, C., et al. Temporal patterning of neuroblasts controls notch-mediated cell survival through regulation of hid or reaper. Cell. 158, 1173-1186 (2014).
- Pinto-Teixeira, F., et al. Development of Concurrent Retinotopic Maps in the Fly Motion Detection Circuit. Cell. 173, 485-498 (2018).
- Plevock, D. A., Rusan, K. M. Live Imaging of Drosophila Larval Neuroblasts. Journal of Visualized Experiments. , e51756 (2014).
- Ting, C. Y., et al. Analyzing dendritic morphology in columns and layers. Journal of Visualized Experiments. , e55410 (2017).
- Özel, M. N., Langen, M., Hassan, B. A., Hiesinger, P. R. Filopodial dynamics and growth cone stabilization in Drosophila visual circuit development. eLife. 4, (2015).

