Determining Temperature Preference of Mosquitoes and Other Ectotherms
Summary
Insects have an optimal environmental temperature range which they seek to remain within, and many external and internal factors can alter this preference. Here, we describe a cost-effective and simple method to study temperature choice, which allows insects to freely exhibit their natural behaviors.
Abstract
Most insects and other ectotherms have a relatively narrow optimal temperature window, and deviation from their optima can have significant effects on their fitness, as well as other characteristics. Consequently, many such ectotherms seek out their optimal temperature range. Although temperature preferences of mosquitoes and other insects have been well studied, the traditional experimental setup is performed using a temperature gradient on an aluminum surface in a highly enclosed space. In some cases, this equipment restricts many natural behaviors, such as flying, which may be important in preference selection.
The objective of this study is to observe insect preference for air temperature by using a two-chamber apparatus with sufficient room for flight. The two chambers consist of independent temperature-controlled incubators, each with a large aperture. The incubators are connected by these apertures using a short acrylic bridge. Inside the incubators are two netted cages, linked via the apertures and bridge, allowing the insects to freely fly between the different conditions. The acrylic bridge also acts as a temperature gradient between the two incubators.
Due to the spacious area in the cage and easy construction, this method can be used to study any small ectotherm and/or any manipulation which may alter temperature preference including sensory organ manipulation, diet, gut flora, and endosymbiont presence at biosafety levels 1 or 2 (BSL 1 or 2). Additionally, the apparatus can be used for the study of pathogen infection using further containment (e.g., inside of a biosafety cabinet) at BSL 3.
Introduction
Organisms can live and reproduce only within their thermal tolerance range. As environmental temperature varies due to seasons changing and global warming, species must adapt and respond accordingly to ensure their survival. This includes ectotherms, where the body temperature is in equilibrium with the environment1. Hence, each insect has their own optimal environmental temperature range which they seek to remain within2.
Temperature is one of the important factors used to predict the distribution and range of insects3,4,5, observing pathogen-insect relationships6,7 and the effect of external factors on the fitness of ectotherms such as their adult lifespan, fecundity, and feeding rate8,9.
Previous studies have investigated the preferred temperature of ectotherms with different setups. The most common is using a large aluminum block either with a cooled or heated water bath10, an ice bath and programmable heater element11, cold and hot plates12,13, thermal regulator plates14,15, or a heat pack and ice pack16 at either end to create a temperature gradient. Additionally, other studies have also used a temperature gradient incubator to study the growth of selected bacteria17 and mounted an aluminum rod on a thermoelectric device (heated and cooled at the ends) to observe the thermal preference of Drosophila melanogaster18,19.
However, the alternative methodology proposed here has significant advantages for certain insect applications. Firstly, other solutions require complete construction from scratch with basic materials, including aluminum sheets, constructing acrylic chambers for the insects, and often a camera setup and specialist software; this can be expensive and time-consuming to set up. Secondly, many alternative apparatuses rely on a temperature gradient on a surface (as opposed to air temperature). Consequently, the chamber in which the insects are studied is often very narrow (e.g., 24 cm long gradients with only 2 cm width and 1 cm depth16), which may prevent natural behaviors, such as flight, which are essential for the normal mobility of insects and hence imperative in selecting a preferred temperature. Some studies do measure the air temperature; however, the scoring of choice still involves counting the number of mosquitoes landing on the Peltier elements as opposed to insects flying freely in the cages20.
In this study, we describe a simpler setup, which uses minimally modified standard equipment and provides insects with sufficient room to fly and navigate relatively unhindered in a standard-sized colony maintenance cage. Further, rather than relying on a gradient, the protocol utilizes two relatively large-sized sections of consistent internal temperature, allowing for natural roaming of the insects at their preferred temperature and a simple binary scoring. Hence, the apparatus and protocol described here provide a low-cost and simple means of studying mosquito temperature preference in a less obstructive and more realistic setting.
The protocol involves preparation of the insects before the experiment followed by the two-chamber apparatus setup. Further steps include placing insects in the apparatus to allow the choice of temperature and scoring of results. For an illustration of the method here, we chose the optimal (standard rearing) temperature of the insects, 27 °C for Aedes aegypti, 25 °C for Drosophila melanogaster, and a higher repelling temperature for both species of insects, 30 °C and 28 °C, respectively. Insects are given 30 min to select a preferred chamber. This time was found to be sufficient, and a longer duration did not change the results; however, this may be extended depending on species/temperature/other variables as needed.
Protocol
NOTE: This protocol is written for BSL 1 or 2; for BSL 3 work, perform the entire protocol inside a class 3 biosafety cabinet (glove box).
1. Insect preparation
- Prepare two empty mosquito cages (17.5 cm x 17.5 cm x 17.5 cm) with 12 cm of sleeve openings (Figure 1). Before proceeding with the experiments, ensure there are no holes or other damage to the mosquito cages.
- Using a mechanical aspirator (a simple pooter with a collection chamber), transfer 30 insects (e.g., Aedes aegypti mosquitoes; here, females 3-5 days post-emergence were used), to a separate cage for easier handling and disposal after the experiment.
NOTE: A total of 30 insects per experiment is suggested as it is easy to manage and count without a high risk of mosquito escape. The number of insects used can be adjusted to fit the objective of the experiment.
2. Two-chamber apparatus setup
- Set the incubators to the desired temperatures, as per the incubator manufacturer's instructions.
- Allow the incubators to heat up and stabilize at the specific temperatures, which is <30 min for temperatures in the range of 25-30 °C. Check the air temperature in the incubator with a temperature probe, to ensure the incubator is set up to the intended temperature.
- Place an empty mosquito cage in each incubator (Figure 2A).
- Feed the sleeves of the cage through the front hole of the incubator. Prepare an openable cover (flap) with duct tape and place it over the hole in the acrylic tube (Figure 2B).
- Insert the acrylic tube into the sleeve of one cage on top of the incubator hole. The diameter of the tube is larger than the hole in the front of the incubators so that it completely covers the hole.
- Tighten the mesh of the sleeve around the tube with a rubber band or reusable cable tie (Figure 2C). Ensure that the acrylic tube is not loose and dangling between theincubators; if it is, pull the cage sleeves to remove excess material between the cage and rubber band.
- Place both incubators facing each other and repeat steps 2.5 and 2.6 with the sleeve of the other incubator. Both cages are now securely linked through the acrylic tube (Figure 2D).
3. Mosquito insertion
- Open the duct tape flap for mosquito insertion. Place a funnel into the hole. Empty the insects into the funnel that has been placed in the acrylic tube.
NOTE: If desired/required: for mosquitoes, use a CO2 pen to knock out all mosquitoes before placing them in the funnel21; for Drosophila, use ice to knock insects down22. - Remove the funnel and cover the hole in the tube with the duct tape flap. Leave for 30 min for insects to select the preferred chamber.
NOTE: If CO2 or ice was used, lightly tap the tube bridge to wake the insects after a couple of minutes.
4. Mosquito counting
- After 30 min, visually observe and write down how many insects were found in the bridge (the acrylic tube).
- Tap/blow the insects in the bridge to either side of the incubator. Record to deduct from the total number of insects later on.
NOTE: Knock out all 30 insects in the apparatus by releasing CO2 into the bridge (use CO2 for all insects as ice will not knock down insects in the cages). Also, take note of the number of insects in the bridge which fly to either side of the incubator. - Pinch and close the sleeves from the acrylic tube on both sides, quickly fasten with a knot to close the cages, and ensure that the rubber band is still intact to prevent any insects from escaping.
- Remove the cages from the incubator, and visually count the insects in each cage (deduct the number of insects from the bridge if needed).
- Repeat step 4.4 with the other cage. Make sure that the numbers from the two incubators and the bridge add up to 30 (or the number of insects used, if different).
- If the numbers do not add up to the total number of insects used in step 1.2, look for the remaining insects in the cage sleeve.
5. Replication
- When performing experiments, be sure to account for possible external biases, such as light direction, ambient smells, etc. For example, by reversing the cages, incubator orientation and combinations between replicates.
Representative Results
In order to test the efficacy and effectiveness of this experimental setup, 30 mosquitoes were tested with the same temperature in both incubators in four replicates (Figure 3). When both chambers were set to the mosquito optimal temperature of 27 °C, there was no significant difference between chamber preference (P = 0.342; Wilcoxon signed-rank test). However, when one chamber was set to the attractive optimal temperature of 27 °C and the other chamber to a sub-optimal temperature of 30 °C, mosquitoes consistently demonstrated active preference toward their optima (P = 0.029; Wilcoxon signed-rank test; mean value of 78.2% and 21.8% for 27 °C and 30 °C, respectively). We also tested using Drosophila to determine the applicability with another ectotherm model and similar results were observed.
Temperature uniformity within cages
Figure 4 shows the temperature uniformity of the two-chamber apparatus. Once assembled, the two sides were set to 27 °C and 30 °C and allowed to equilibrate as per the instructions given here. All parts of the incubator and bridge are within 0.4 °C of the central temperature, except (consistently) for one corner. Note, that the front bottom left-hand corner (as viewed from the front) is a consistent hot spot at both 27 °C and 30 °C. This is likely due to the electronics of the incubator controls being situated just beneath that section of the incubator, rather than the manipulations performed; hence, it is likely incubator model-specific. This demonstrates that the manipulation and addition to the incubator have minimal effect on the temperature uniformity. Furthermore, the bridge temperature was intermediate between the two chambers, ensuring that insects are not confronted with a temperature trough that they would have to fly through.

Figure 1: Description of the mosquito cage. Mosquito cage (17.5 cm x 17.5 cm x 17.5 cm) with 12 cm sleeve openings. Please click here to view a larger version of this figure.
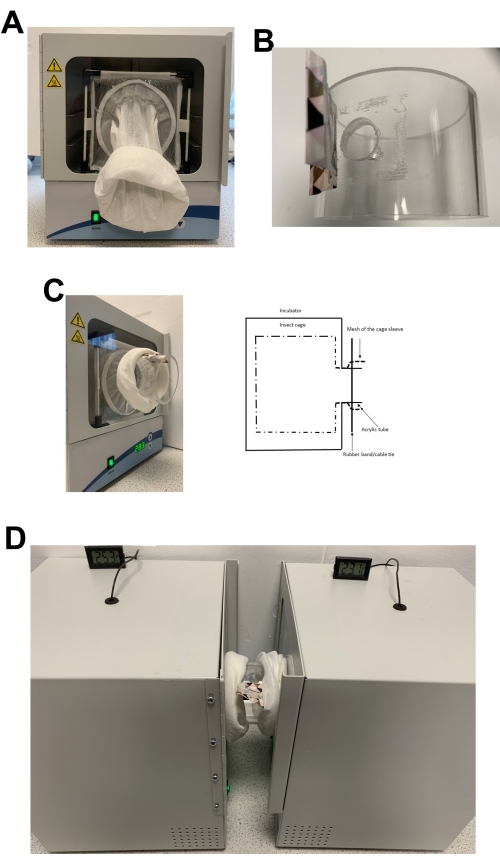
Figure 2: Pictures and diagram of the apparatus during setup. (A) Empty insect cage placed in the incubator. (B) Acrylic tube with an openable cover (flap) made from duct tape. (C) Side view of the setup with a schematic diagram. The mesh of the sleeve was tightened around the acrylic tube with a rubber band. For these experiments, 3-5 day old, mated, female Ae. aegypti mosquitoes were used. (D) Complete setup. Two incubators facing each other are connected by an acrylic tube. Please click here to view a larger version of this figure.

Figure 3: Temperature preference in insects. The two-chamber apparatus was assembled as per the instructions. Insects were inserted as per the protocol and left for 30 min to select their preferred chamber (temperature) and then counted. Black points represent individual replicates, and blue represents the mean. (A) Both incubators were set to the same temperature (27 °C) and the temperature preference of Ae. aegypti was observed. (B) Incubators were set to different temperatures (27 °C vs. 30 °C) and the temperature preference of Ae. Aegypti was observed. (C) Incubators were set to different temperatures (25 °C vs. 28 °C) and temperature preference of D. melanogaster was observed. Please click here to view a larger version of this figure.
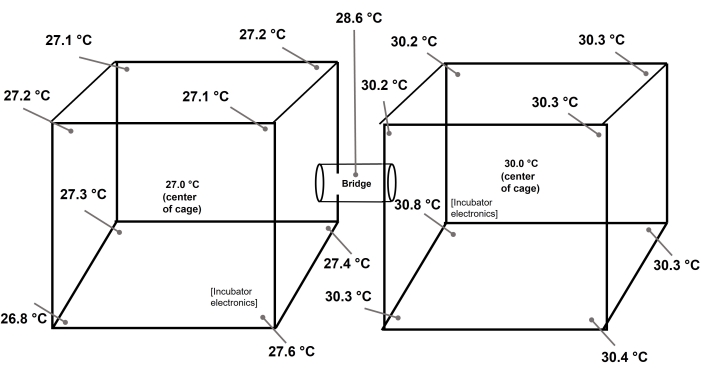
Figure 4: Temperature uniformity within the chambers and bridge. As described, two incubators, two cages, and the bridge were assembled as per the instructions. The temperature was adjusted to 27 °C on both incubators and 30 °C in the center. A temperature probe was used to measure the temperature in the center of the cage, all eight corners of the incubator, and inside of the bridge. The temperatures measured are shown here. Please click here to view a larger version of this figure.
Discussion
The study describes a new method to observe the temperature preference in mosquitoes. In this method, mosquitoes are released into a tube that is connected to two incubators with independently controllable temperatures. In this manner, the mosquitoes are allowed to freely choose between two temperatures without disrupting their natural behaviors and mechanism of expressing this choice (e.g., flying).
Our first representative experiment used the mosquito optimal temperature of 27 °C in both chambers. During the repetitions of this experiment, mosquitoes were observed to be freely flying between both cages for the entire 30 min, and in all replicates, there were near equal numbers in each of the two chambers. This confirmed the experimental intention of allowing the mosquitoes the ability to freely choose between cages while exhibiting their natural behaviors (flying). Conversely, the second representative experiment utilized the attractive optimal temperature of 27 °C in one chamber and a sub-optimal and hence repelling temperature of 30 °C in the second chamber. As expected, mosquitoes consistently selected the optimal temperature chamber at high significance, even when we swapped the incubators to avoid bias.
We also tested the setup for a different insect, D. melanogaster (fruit flies), representing another ectotherm model organism. One chamber was set to the optimum temperature of D. melanogaster, 25 °C, and the other was set to 3 °C higher, 28 °C. Similar to mosquitoes, fruit flies also favored their optimal temperature and avoided the warmer chamber. This demonstrates that the protocol is suitable for a range of ectotherms.
Description of critical steps in the protocol
The main critical step in the protocol is insect handling, as it generates the possibility of insects escaping. This can be prevented by determining that there are no holes large enough for escape in the cages used, that the rubber bands/cable ties used to secure the mesh sleeves to the bridge are tight, and that the cover for the insect insertion hole on the bridge is securely attached and sealed.
It is also crucial to ensure insects do not escape before or after the experiment, particularly when the insects are required for downstream experimentation or later time points for various temperature choices. This can be done by anesthetizing the insects before placing them in the acrylic bridge (using ice for Drosophila and CO2 for mosquitoes) and releasing CO2 into the bridge to knock down the insects after the experiments, prior to calculating. The usage of CO2 is ideal for mosquitoes since it won't affect the behavioral results21. In flies, exposure to CO2 can alter their flying behaviour23, hence it is recommended to use ice22.
Counting of insects is also a critical step, to ensure the numbers of insects are equal before and after the experiment for accurate results. In order to do this, we recommend the usage of a CO2 pen once the experiment is completed to knock down the insects that are located in the bridge. This will help move the insects to either side of the chamber, hence reducing the number of escapees. We also highlight in the protocol that insects can be caught in the sleeves of the cages during cage separation; therefore, ensure these are checked thoroughly during counting.
Potential modifications and troubleshooting of the technique
The main difficulty with this technique is the flexible mesh of the cage sleeves resulting in gaps or hiding places and hence insect escape or trapping. There are some potential modifications, if needed, to improve the technique. We suggest using two or more rubber bands to ensure that the bridge is secured properly in between the chambers without leaving any potential space for the insects (loose mesh creates a hiding space for insects). We also advise particular care to pull the mesh sleeve taut, as described in step 2.6, when assembling the apparatus.
Small form factor incubators are usually heated only (i.e., have no active cooling), as was the case for the incubators used here. Consequently, using temperatures around or below the ambient room temperature will require the experiment to be performed in a cold room to ensure that the temperatures set for the incubators will go as low as desired.
In addition, this setup can also be used for BSL 3, where a class three biosafety cabinet (glove box) is needed. In this case, the glove box needs to be big enough to fit the entire apparatus. The experiment described in this protocol is ideal for experiments in a glove box because everything required will be contained within the glovebox and, importantly, the possibility of insects escaping is minimal.
Finally, there is enough space in the incubators to add external light or a humidity source without affecting the insects in the cages. Depending on the insect species or experimental design, an LED lamp with 1 cm thickness can be easily placed on top of the cage inside one or both incubators. Providing light to both and offering a temperature choice can be a more realistic protocol for some photosensitive experimental designs, or only providing light (or humidity) to one chamber is a possible modification to the protocol to assess light/humidity choice.
Advantages of this technique in the context of dual choice temperature preference assays
The method described here presents an alternative to the traditional temperature gradient method described in previous studies10,13,14,16. In most of these studies, a large horizontal aluminum block with a thermal gradient is used, while the mechanism of generating this gradient varies, including heating/cooling blocks, water baths, etc. In these instances, the temperature gradient is produced on the surface of the aluminum block (rather than the air temperature in a cage). Consequently, most (but not all) alternative techniques do restrict the flight ability of insects more than this protocol. Here, insects can fly relatively freely between cages, allowing for a more realistic expression of natural behaviors in choice. It would even be possible to scale-up this experimental apparatus using larger cages and incubators, for example, for larger insects.
In addition to the natural behavior advantage, we also demonstrate very high temperature uniformity within the two chambers, enabling simple scoring and a clear selection of two large single temperature chambers. The use of a binary large chamber design such as this may reduce noise in the data, where, for example on a gradient apparatus, any incidental movement of the insects will alter position on the gradient and hence their perceived temperature preference.
The technique described here is also very simple and low cost. This technique does not need extra appliances to set the temperatures (i.e., a water bath10 and/or a hot plate11,12,13,14,15), no specialist equipment besides a cut acrylic tube and drilled holes, and no camera18,19 or sophisticated software19 for analysis. Such components used in other techniques can be expensive and/or require significant expertise and testing to begin experiments.
This technique can also be replicated with different devices that use batteries if there is no external power supply, making the system ideal to conduct experiments in the field. Furthermore, the same apparatus could be slightly modified to study other binary choice preference situations, such as light versus dark, high/low humidity, etc., either in the laboratory or field.
The full-sized apparatus in the protocol is significantly smaller than temperature gradient setups, allowing for an easier fit inside a BSL 3 glovebox as described above. Further, the insects are easier to contain, as they can be knocked down with CO2 at the end of the experiment, and the cages can be quickly resealed after separation from the bridge. These containment advantages are ideal for BSL 3 work.
We do however acknowledge that our apparatus only allows for a binary decision rather than a free choice along a gradient, which, depending on the application, may require additional runs to identify optimal temperatures.
Divulgations
The authors have nothing to disclose.
Acknowledgements
AHR acknowledges funding support from Majlis Amanah Rakyat (MARA).
Materials
| Acrylic tube (Bridge) | Perspex | 900 mm OD | Size (Length x diameter): 8 cm x 9 cm; 1 cm bigger than the size of the hole in front of the incubator. Size of the hole on top: 1.6 cm |
| Carbon dioxide (CO2) inflator | Peaken | B08HM2BDDB | Any CO2 pen will work |
| Digital Incubator (×2) | VWR | VWR INCU-Line 1L 10 (390-0384) | Size of hole in front of incubator: 8 cm diameter. Holes need to be position in the center and have the same exact position on both incubators to allow alignment of bridge.This should be pre-drilled using a standard 8 cm ‘holesaw’ drill bit. Incubator must be just large enough to contain one mosquito cage. |
| Mechanical aspirator (for mosquitoes) | Watkins and Doncaster | E710 | Ideal barrel size 50 x 28 mm and tube diameter 9mm. |
| Mosquito cage (×3; two for the experiments, one for storing insects) | BugDorm | BD4S1515 | Size: 17.5 cm x 17.5 cm x 17.5 cm with 12 cm sleeve opening. Mesh material : Knitted nylon |
| Plastic funnel | Diameter of opening = 5 cm Length of funnel = 5 cm Diameter of aperture = 1 cm |
||
| Plastic Pocket Pooter (for Drosophila or small insects) | Watkins and Doncaster | E714 | Manual/mouth aspirated |
| Rubber band or Reusable cable tie | Either, depending on preference. | ||
| Temperature probe | Eidyer | B07J4T1VQZ | Any thermometer with at least 100 cm narrow wire probe |
References
- Wright, R. K., Cooper, E. L. Temperature effects on ectotherm immune responses. Developmental & Comparative Immunology. 5, 117-122 (1981).
- Deal, J. The temperature preferendum of certain insects. The Journal of Animal Ecology. 10 (2), 323-356 (1941).
- Hongoh, V., Berrang-Ford, L., Scott, M. E., Lindsay, L. R. Expanding geographical distribution of the mosquito, Culex pipiens, in Canada under climate change. Applied Geography. 33, 53-62 (2012).
- Beck-Johnson, L. M., et al. The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk. Royal Society Open Science. 4 (3), 160969 (2017).
- Erraguntla, M., et al. Predictive model for microclimatic temperature and its use in mosquito population modeling. Scientific Reports. 11 (1), 18909 (2021).
- Shapiro, L. L., Whitehead, S. A., Thomas, M. B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biology. 15 (10), 20033489 (2017).
- Zhang, Y., et al. Decline in symbiont-dependent host detoxification metabolism contributes to increased insecticide susceptibility of insects under high temperature. The ISME Journal. 15 (12), 3693-3703 (2021).
- Amarasekare, P., Savage, V. A framework for elucidating the temperature dependence of fitness. The American Naturalist. 179 (2), 178-191 (2012).
- Buckley, L. B., Nufio, C. R. Elevational clines in the temperature dependence of insect performance and implications for ecological responses to climate change. Conservation Physiology. 2 (1), 035 (2014).
- MacLean, H. J., et al. Temperature preference across life stages and acclimation temperatures investigated in four species of Drosophila. Journal of Thermal Biology. 86, 102428 (2019).
- Castañeda, L. E., Romero-Soriano, V., Mesas, A., Roff, D. A., Santos, M. Evolutionary potential of thermal preference and heat tolerance in Drosophila subobscura. Journal of Evolutionary Biology. 32 (8), 818-824 (2019).
- Weldon, C. W., Terblanche, J. S., Bosua, H., Malod, K., Chown, S. L. Male Mediterranean fruit flies prefer warmer temperatures that improve sexual performance. Journal of Thermal Biology. 108, 103298 (2022).
- Sayeed, O., Benzer, S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proceedings of the National Academy of Sciences. 93 (12), 6079-6084 (1996).
- Verhulst, N. O., Brendle, A., Blanckenhorn, W. U., Mathis, A. Thermal preferences of subtropical Aedes aegypti and temperate Ae. japonicus mosquitoes. Journal of Thermal Biology. 91, 102637 (2020).
- Ziegler, R., Blanckenhorn, W. U., Mathis, A., Verhulst, N. O. Video analysis of the locomotory behaviour of Aedes aegypti and Ae. japonicus mosquitoes under different temperature regimes in a laboratory setting. Journal of Thermal Biology. 105, 103205 (2022).
- Blanford, S., Read, A. F., Thomas, M. B. Thermal behaviour of Anopheles stephensi in response to infection with malaria and fungal entomopathogens. Malaria Journal. 8, 72 (2009).
- Nakae, T. Temperature-related anomalies in the growth of selected bacteria. Journal of Dairy Science. 54 (12), 1780-1783 (1971).
- Rajpurohit, S., Schmidt, S. P. Measuring thermal behavior in smaller insects: A case study in Drosophila melanogaster demonstrates effects of sex, geographic origin, and rearing temperature on adult behavior. Fly. 10 (4), 149-161 (2016).
- Truitt, A. M., Kapun, M., Kaur, R., Miller, W. J. Wolbachia modifies thermal preference in Drosophila melanogaster. Environmental Microbiology. 21 (9), 3259-3268 (2019).
- Reinhold, J. M., et al. Species-specificity in thermopreference and CO2-gated heat-seeking in Culex mosquitoes. Insects. 13 (1), 92 (2022).
- Lin, C. S., Georghiou, G. P. Tolerance of mosquito larvae and pupae to carbon dioxide anesthesia. Mosquito News. 36 (4), 460-461 (1976).
- Ito, F., Awasaki, T. Comparative analysis of temperature preference behavior and effects of temperature on daily behavior in 11 Drosophila species. Scientific Reports. 12 (1), 1-15 (2022).
- Bartholomew, N., Burdett, J., VandenBrooks, J., Quinlan, M. C., Call, G. B. Impaired climbing and flight behaviour in Drosophila melanogaster following carbon dioxide anaesthesia. Scientific Reports. 5, 15298 (2015).

