Intraductal Delivery and X-ray Visualization of Ethanol-Based Ablative Solution for Prevention and Local Treatment of Breast Cancer in Mouse Models
Summary
A method of intraductal injection of reagents for an ethanol-based ablative solution to the mouse mammary ductal tree for in vivo imaging and breast cancer prevention is described. Injection directly into the nipple opening allows for targeting mammary epithelial cells with minimal collateral tissue damage.
Abstract
Breast cancer is the most prevalent cancer and the second-leading cause of cancer-related death for women in the USA. For high-risk women, prophylactic mastectomy is the most effective primary prevention strategy. Prophylactic mastectomy is an aggressive surgical procedure that completely removes the mammary epithelial cells from which breast cancer arises along with the surrounding tissue. We seek to develop a minimally invasive intraductal procedure as an alternative to prophylactic mastectomy to locally ablate the mammary epithelial cells before they can become malignant. We and others have developed an intraductal delivery procedure to reach and treat these epithelial cells in rodent models of breast cancer. While the mouse mammary gland with a single non-anastomosed ductal tree opening at the nipple has a much less complex and tortuous architecture than the human breast, chemically induced and genetically engineered mouse models of breast cancer are valuable to produce proof-of-concept studies of new preventative strategies. Here, we describe a procedure for intraductal delivery of an ethanol-based ablative solution containing micro-CT/X-ray tantalum-based contrast agent within the mouse mammary ductal tree for the therapeutic purpose of primary prevention of breast cancer. Intraductal delivery of aqueous reagents (e.g., cytotoxic compounds, siRNAs, AdCre) has been previously described in mouse models. Thus, we focus our protocol description on methodological modifications and unique experimental considerations for optimizing delivery of ethanol, for minimizing local and systemic side effects of ethanol administration, and for in vivo visualization of ductal tree filling via micro-CT/fluoroscopy imaging. Visualization of the ductal tree immediately after injection of a contrast-containing solution allows for confirmation of complete filling or unsuccessful outcomes such as underfilling or overfilling. This procedure can be applied for delivery and imaging of other ablative compounds aimed at either preventing tumor formation or locally treating early-stage tumors accessible via the ductal tree.
Introduction
Breast cancer is a common and potentially lethal disease with few options available for prevention1. The most effective intervention is prophylactic mastectomy; however, only high-risk individuals opt to undergo this procedure as it is a surgery with major life-changing consequences2. The procedure completely removes the mammary epithelial cells from which breast cancer arises along with the surrounding tissue. This can result in physical, psychological, and social stress for the individual, and often dissuades individuals from proceeding with this surgical procedure as their first line of primary intervention.
We have demonstrated that delivery of an ablative solution containing 70% ethanol (EtOH) directly into the ductal tree is effective at killing mammary epithelial cells with limited collateral tissue damage and at preventing breast tumors in mouse models3. EtOH has been long used clinically as an ablative or sclerosing agent for local treatment. Percutaneous EtOH injection is used as an ablative agent for unresectable liver tumors, renal and adrenal neoplasms, and pancreatic cystic tumors4,5,6; for celiac plexus neurolysis to reduce pain7; and for treating breast pseudoaneurysms8. Intravascular EtOH injection is used as a sclerosing agent to eliminate swelling and deformation from arteriovenous malformations (AVM), and for cosmetic treatment of spider veins and varicose veins9,10,11,12,13. Like prophylactic mastectomy, the success of prevention with local delivery of an ablative solution hinges on the ability to completely remove all mammary epithelial cells from which cancer could potentially arise. This requires confirmation that the ablative substance has successfully filled the ductal tree, thus contacting all mammary epithelial cells directly. Clinical means for injecting substances within the mammary glands and visualizing them by image-guided fluoroscopy or ductography are readily available14,15; therefore, it will be possible to both deliver and confirm successful delivery when this procedure may warrant evaluation in clinical trials.
Demonstrating the feasibility of this image-guided approach in laboratory animals is a key step in establishing the efficacy and translational feasibility of intraductal (ID) ablation as a preventative measure for breast cancer. In our laboratory, we have developed a method to successfully inject all mammary glands in mice with an ablative solution containing a contrast agent over a course of weekly injections to ensure the animal does not succumb to an overdose of EtOH (Figure 1, Figure 2, reference nos.3,16). This procedure places a 34 G needle inside the nipple opening of an isoflurane-anesthetized mouse to inject the test solution. Some key improvements of the procedure include the use of gastight syringes for liquid and gases, injection of higher volumes per ductal trees17, and extended anti-inflammatory treatment. The preclinical treatment of 5 mg/kg of carprofen, an NSAID, from 2 d before to 7 d after the ID procedure is in line with that of clinical sclerosing therapy for AVM. Typically, after systemic anesthesia, the patients receive anti-inflammatory medications, such as NSAIDs, for 2 days post-procedure that can be extended to mitigate any local inflammation or pain12. Alcohol intoxication is significantly mitigated by intraperitoneal injection of a 5% sucrose solution in mice. With administration of this sucrose solution, mice can be safely injected with up to 160 μL of 70% EtOH (up to four ductal trees; about 0.4 g/dL of EtOH content in blood); animals fully recovered within 4 h after ID injections. For injection of more than four glands in mice and/or higher EtOH concentrations, we perform sequential sessions to allow enough recovery time. Alcohol intoxication in women would be a lesser concern due to the lower proportion of alcohol amount to body weight. Given the number of ductal trees in human breast14,15, about 16, and estimated volume to fill each tree duct18,19, up to 32 mL of 70% EtOH will be administered. This quantity will be much lower than the 50 mL of 95% EtOH administered in other clinical procedures4,9. Intravenous administration of thiamine and glucose solution could be used to further minimize effects of EtOH intoxication, especially in cases where a larger total volume of EtOH may need to be injected and/or for women who have a lower tolerance to alcohol consumption (e.g., allelic variants in alcohol or aldehyde dehydrogenases).
Imaging via micro-CT/fluoroscopy allows us to confirm successful ductal filling of each gland (Figure 1, Figure 2, Figure 3). This can be recorded for future analysis, or assessed in the moment via real-time fluoroscopy imaging, as would be done in clinical application, to limit overall radiation burden imposed on the animal. To further improve specific features of this ablative solution for real time image-guided delivery in vivo, we previously compared FDA-approved iodine-containing contrast to a tantalum oxide (TaOx)-containing nanoparticle synthesized by the Shapiro lab3,16. TaOx showed superior performance as a micro-CT contrast agent for visualizing the initial filling of the ductal tree (Figure 2, Figure 3). TaOx can be used as a reference contrast to perform a more systematic and longitudinal assessment of other nanoparticle-based blood pool contrast agents (e.g., iodine-, bismuth-, or gold-containing) and compatibility of TaOx with different concentrations of ethyl cellulose as gelling agent20,21.
Protocol
All the experiments described here were conducted under protocols approved by Institutional Animal Care and Use Committee at Michigan State University.
1. Extended anti-inflammatory treatment
- Ensure that mice receiving injection solutions containing EtOH or other potential irritants are provided with anti-inflammatory treatment from 2 days before injection to 7 days after injection. The preferred method of dosing is oral delivery through a sucralose gel cup containing carprofen at an appropriate concentration.
- Prepare carprofen with the required concentration. For this experiment, a working solution of 2 mg/mL (Stock solution is 50 mg/mL) was prepared by diluting in sterile PBS to inject 0.5 mL to achieve a final dose of 1 mg per cup. Add 1% v/v sterile blue food dye (BFD) in place of a fraction of the PBS of the total volume needed for dilution to better visualize complete mixing of the drug into the sucralose gel.
- Prepare a cup for carprofen addition as per the manufacturer's recommendation. Unless recommended otherwise, place the cup in 60 °C water bath for 15 min. Remove the cups and dry them off to minimize the possibility of contamination.
- Use 70% EtOH or an EtOH wipe to clean the surface of the cup lid and allow to dry. Use a syringe to inject the necessary amount of carprofen solution through the lid. For this experiment, 500 µL is injected. Cover the injection site with a sticker and shake vigorously for 15 s.
- Vortex the cup for 15 s, and then store for later use after visually verifying complete mixing. Check the cups for homogenous mixing by looking for dark blue clumps. Allow the cups to come to room temperature before moving to the refrigerator for storage up to one month.
NOTE: These cups can also be stored at room temperature once dosed, if needed, but take care to pay attention to drug efficacy guidance from the manufacturer. Dating the sticker helps with keeping track of the injection date without risking a pen or sharp marker puncturing the lid.
- When ready to use, wipe down the exterior of the cup with 70% EtOH. Peel off the lid and place the cup into the cage with the mice. Replace the cups every other day or when empty. One cup should be enough for up to five mice for 1-2 days.
2. Preoperative preparation
NOTE: This step will occur 2-3 days prior to the injection.
- Anesthetize the mouse using an isoflurane vaporizer (2%-3% isoflurane, 1.5 L/min of oxygen) and apply eye lubricant. Maintain anesthesia at 1%-3% isoflurane as needed throughout the procedure with a nose cone while carefully monitoring the respiratory rate of the animal.
- Apply the eye lubricant to the mouse eyes while at the nose cone, and then position the mouse on its back. Use a cotton tipped applicator to apply over-the-counter depilatory cream to the nipple area (the area of the mammary gland that will be injected). Rub the cream into the area for 10-30 s with the applicator to help loosen the fur quickly.
NOTE: Do not leave the cream on the animal any longer than necessary and remove completely to avoid burning the skin. - After 10-30 s of the application, use warm water on gauze to completely remove the cream and loosen the fur from the animal. Perform at least two to four rinses of the area with fresh gauze for cream removal before drying with a clean dry gauze after the final rinse. Check the area of fur removal to confirm good visibility and access to the nipples. Repeat with depilatory cream application if necessary.
- Place the mouse in a separate clean, dry recovery cage on a heating pad to recover from anesthesia. Observe the mouse until it has fully recovered from anesthesia and return it to the home cage.
- Provide mice with one cup of sucralose gel solution containing carprofen (1 mg/cup) for pre-dosing of the anti-inflammatory agent after recovery. One cup can provide up to five mice with carprofen for up to 2 days. Check the cup daily and replace as needed, or every other day if not fully consumed.
3. Intraductal injection
NOTE: This step will occur 2-3 days after preoperative preparation.
- Prepare 333.3 mM tantalum oxide (TaOx) stock solution from procured powder formulation as described in16 using phosphate buffered saline (PBS). Use gentle warmth if the powder does not fully dissolve into the solution.
- Make an ablative imaging solution by mixing three parts stock TaOx with seven parts 100% EtOH for a final 70% EtOH 100mM TaOx solution. Add 1% v/v blue food dye to the final solution for aided visualization during injection. Prepare a volume based on need for the experiment.
NOTE: Gland pairs 1 and 5 can hold up to 30 µL of the solution while all other pairs can be injected with up to 50 μL in 9 weeks of age or older FVB mice. - Anesthetize the mouse with isoflurane as in preoperative preparation and move the mouse to the nose cone once fully anesthetized. Apply eye lubricant before placing the animal on its back for the injection. Secure the mouse beneath the stereoscope using tape, if needed.
- Prepare the syringe with the desired volume of injection solution. Load 21-51 µL of the injection solution into a 50 µL syringe with a 34 G needle attached. Load an extra 1 µL of the solution to account for the potential leakage of that volume after the needle is removed from the nipple.
NOTE: Volumes above are for the typical procedures presented here. One may inject any volume desired with the understanding that most glands will be overfilled if going above 30 μL or 50 μL in gland pairs 1 and 5, or 2-4, respectively. It is helpful to fill the needle with additional volume of the injection solution to test for the free flow of the solution immediately prior to the injection. If injecting more than one mammary gland, prefill multiple syringes to save time. However, do not leave the solution in the syringe for long. - Prepare the nipples for the injection by removing any dead skin that covers the nipple opening with fine pointed forceps.
- Hold the nipple gently with tweezers. With the bevel of the needle visible, insert the needle into the nipple opening until the bevel is fully covered with guidance help from fine tipped forceps. It may be necessary to pull up the nipple onto the needle rather than pushing the needle into the nipple (Table 1).
- Once the needle bevel is fully surrounded by the nipple and is in the main duct, begin injecting the solution at a steady rate of approximately 40 µL/min. Avoid injecting too quickly to ensure that the ductal tree is not damaged. Keep the needle in the nipple for 30 s after the volume is completely injected before removing the needle with assistance using the forceps. This is done to avoid the solution spilling out of the nipple (Figure 2).
- Assess the area for any signs of unsuccessful injection. A domed appearance may indicate a fat pad injection or trauma to the area.
- Proceed to inject the remaining mammary glands.
- While the animal is still anesthetized, inject 200-250 µL of 5% sucrose solution (up to 10 mL/kg) intraperitoneally to minimize the potential effects of alcohol intoxication.
4. micro-CT imaging
- After injecting all the desired glands, move the animal swiftly to the micro-CT system and continue to maintain anesthesia using the incorporated isoflurane vaporizer. The animal may be taped to standardize the imaging position. For example, taping each hind leg in an extended position helps to keep the leg bones of the animal further from the lower glands of interest in the resultant image.
NOTE: The animals can be imaged using different scan parameters for the visualization of the ductal tree if care is taken to determine an appropriate acceptable lifetime dose of radiation for the animal and the cumulative doses do not exceed this level. Fluoroscopy stills and videos can be generated without performing scans to further reduce radiation exposure (Figure 2). - Position the mouse in the appropriate field of view with the fluoroscopy preview function.
- Perform TaOx imaging of the mouse ductal tree with good resolution and opportunity for repeated standard (2 min) acquisition scans. Use the following scan parameters: 90 kVp/88 µA; field of view (FOV), 36 mm; number of slices, 512; slice thickness, 72 µm; voxel resolution, 72 µm3.
- Acquire longer (4 or 14 min) high resolution scans for even better resolution in animals. These are acceptable as terminal procedures before euthanasia. These are, however, not acceptable for animals to be scanned longitudinally using the same parameters as it will cause radiation sickness.
- Following imaging protocol completion, remove the animal from anesthesia and transfer to a separate clean, dry recovery cage on a heating pad. Observe the animal until fully recovered, and then return it to the home cage. Keep the animals injected with ablative solution on carprofen until 7 days after injection.
- Make quick renditions of any scanned images within the micro-CT software (built in system) to better appreciate any contrast leaks or lack of filling (Figure 2) without formal analysis.
- Perform further formal image analysis for publication or detailed analysis of scans that allow segmentation of the area of interest if desired (Figure 3).
NOTE: The primary difference between these methods will be the ability to threshold only the ductal tree (formal rendition) versus needing to threshold the entire image (quick rendition). Other measurements and images may be generated using the software packages to best demonstrate the success of ductal tree filling.
5. Image analysis
- Perform rendering of the injected ductal tree using a specialized software package. To do this, segment out the mammary fat pad with further image processing.
- To segment the fat pad (darker compartment compared to peritoneal cavity, femoral muscles, and skin) within which the ductal tree of interest is contained, select the Spline Trace option from the manual menu. Trace the fat pad outline at every third data slice.
- Click on Propagate Objects option from the semi-automatic menu to connect all the traced and untraced slices into a single object.
- Select the Threshold Volume option from the semi-automatic menu to input the desired HU range (300-3,000 HU is a good starting point), and then click on Threshold Rendition button to create a rendition that only displays the contrast (TaOx) within the ductal tree and eliminates soft tissue.
- Toggle the View button to Rendition as Primary to view only the 3D rendition without surrounding structures.
NOTE: Additional software features allow for measurements to be made of the 3D rendition (i.e., length, volume, etc.).
Representative Results
Female mice have five pairs of mammary glands with a single ductal tree that opens at the nipple orifice22. At the tips of the developing ductal tree are the terminal end buds (TEBs), proliferative structures that direct growth and branching. After puberty when the elongation phase is completed, TEBs regress and become functionally and anatomically indistinguishable from terminal ducts or alveolar buds23. Terminal ductal lobular units serve a similar function in humans as TEBs do in mice and are the sites from which breast cancer predominantly arises24,25. We can inject up to 50 μL of 70% EtOH solution to fill the entire ductal tree of thoracic and abdominal mammary glands of 9-week-old FVB/N, NSG, and other mouse strains (Figure 1, Figure 2, Figure 3, see references3,16). In a typical experiment, we can inject up to eight mammary glands with an ablative solution of 70% EtOH and 100 mM TaOx in two consecutive ID procedures separated by 7 days to allow for animal recovery (Figure 2). Animals are imaged by micro-CT immediately after the last ID injection to assess successful delivery of the solution to the entire ductal tree (Figure 2). In our experience, the nipples of inguinal glands are suitable for injection in about 60% of the animals, and the nipples of cervical glands in about 40%. When suitable, we can inject up to 30 μL of 70% EtOH solution to fill the entire ductal tree of cervical and inguinal mammary glands (Figure 2). FVB and NSG strains generally present more suitable nipples for injection than C57BL/6J or mixed genetic background strains. Whole mount dual staining protocol or 3D confocal microscopy are good orthogonal methods to confirm to what extent the ductal tree was filled (Figure 3). These tissue-correlate analyses are compatible with and can be performed after in vivo imaging. The obvious limitation of these orthogonal methods is that they require animal termination for tissue collection and analysis; however, in an optimization phase of a new ablative formulation, they provide independent validation.
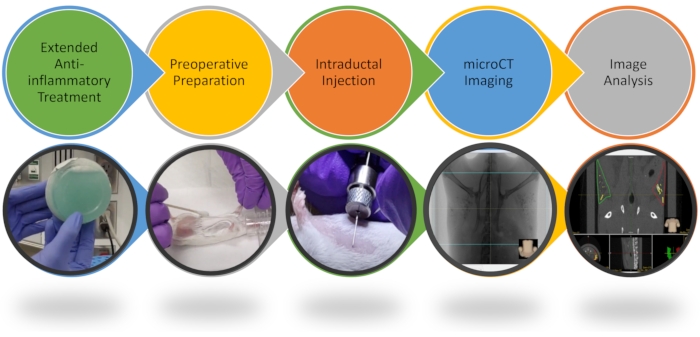
Figure 1: Workflow of intraductal procedure and image analysis. Key steps of the ID procedure are highlighted. Please see the video for more details. Please click here to view a larger version of this figure.
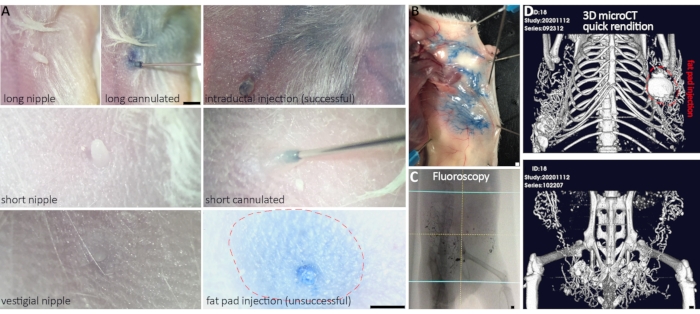
Figure 2: Successful cannulation and delivery of ablative solution to multiple mammary glands. (A) Representative nipple variegation in FVB and NSG mouse strains. Long nipples are easier to cannulate than short nipples, whereas too short or vestigial nipples cannot be cannulated. Once cannulated, the size of the nipple does not affect successful intraductal delivery. (B) Gross anatomical analysis of blue dye in an ablative solution provides ex vivo evidence of ductal tree filling and delivery success. (C,D) Real-time fluoroscopy and post-image acquisition 3D micro-CT rendition provide in vivo evidence of delivery success. (D) Successful injection of both abdominal and inguinal glands, and three out of four thoracic glands (fat pad injection in gland #2). Scale bars correspond to 1 mm in images at different magnification. Please click here to view a larger version of this figure.
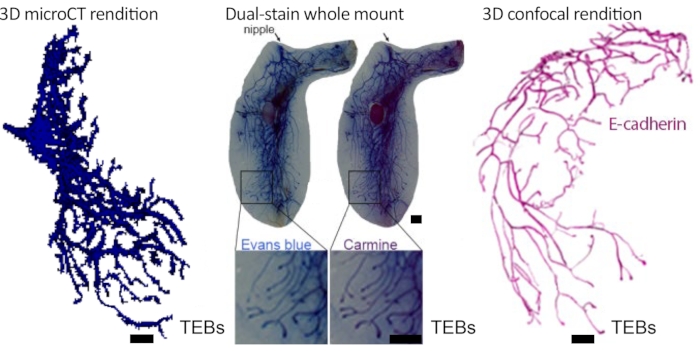
Figure 3: In vivo and ex vivo demonstration of ductal tree filling. 70% EtOH/100 mM TaOx nanoparticles/Evans Blue solution was intraductally injected into the mouse abdominal mammary gland and immediately imaged by micro-CT and processed for dual whole mount staining. Ductal tree was reconstructed using an image analysis software package. The solution entirely fills the carmine alum-stained ductal tree. A separate gland was immunostained for E-Cadherin (Cdh1), cleared using Benzyl Alcohol:Benzyl Benzoate and imaged by confocal microscopy as described26. The ductal tree was reconstructed using image analysis software. Pseudo-coloring rendering of confocal image (i.e., black background to white, green marker signal to magenta) was obtained with the image invert function of the image editing software. Scale bars correspond to 1 mm in images at different magnification. This figure has been modified from reference no.3. Please click here to view a larger version of this figure.
| Issue | Appearance | Solution | |||
| Short nipple (Fig. 2) | Nipple has low profile – hard to grab | It is sometimes easier to hold the skin near the nipple and target the center of the nipple with the needle. The needle will likely dive under the skin. Pulling up slowly may reveal the nipple to be slightly over the tip of the needle and give room to grab and pull it the rest of the way onto the needle. Be very careful when diving below the skin about the angle of the needle. It is easy to inadvertently get a fat pad injection by stabbing at the wrong angle. | |||
| Fat nipple | Much larger than other nipples with little peelable dead skin – easily visible without scope | Very easy to get a fat pad injection on these nipples. Be very cautious about angle of the needle when inserting into nipple. | |||
| Fat pad injection (Fig. 2) | Swollen around nipple and possibly in nipple itself – easiest to see if color is added to injection solution | If nipple is swelling with first few ul injected, remove needle, and attempt to insert again with more care taken of angle. Begin injection again and watch for further swelling. If swelling continues, abandon attempt. It is very rare to successfully inject a nipple that has started out as a fat pad injection. | |||
| Wounds/scabbing | Open wound or scabbing near injection site of EtOH solution | Apply triple antibiotic ointment to open wounds but leave scabbed wounds alone. Applying ointment to scabs can increase likelihood animal will bother the scab and remove it. Check every 1-2 days until healed depending on severity of wound. Carprofen should be given until healed even if beyond normal window. | |||
Table 1: Troubleshooting and helpful tips.
Discussion
Prophylactic mastectomy is currently the most effective intervention for breast cancer, yet it has some serious negative impacts. Local ablation of mammary epithelial cells with an EtOH-based solution is a promising alternative therapy as we demonstrated in a proof-of-concept study on the aggressive FVB-Tg-C3(1)-TAg mouse model of breast cancer3. ID injection of this ablative solution allows for targeting of the mammary epithelial cells from which breast cancer arises with limited collateral damage. Addition of an X-ray contrast agent to the ablative solution allows for enhanced understanding of the effectiveness of the solution at prevention, as we can see whether each ductal tree is successfully filled following injection (Figure 2B). Viewing injected glands by fluoroscopy immediately post-injection mirrors what will likely be done in the clinic to confirm successful filling of the ductal tree. Visual confirmation of solution delivery will best inform whether all parts of the tree have been reached in real time. This could allow for further injection to be performed to complete filling at the time or in a future session. It is of great importance that ablative solution reaches all parts of the ductal tree to ensure that all epithelial cells may be accessed for killing (Figure 3). Leaving behind living epithelial cells within the tree would allow for the possibility that breast cancer could still arise. Utilizing contrast in ID injections to image success of the injection could also be useful for other formulations. Table 1 provides troubleshooting and helpful tips. Other studies have described ID delivery protocols for viral particles (e.g., AdCre, CRISPR guide RNAs), hormones, cytotoxic compounds, siRNAs and/or targeting agents in mice3,16,27,28,29,30,31,32,33,34,35,36,37,38, rats24,32,39,40,41, and rabbits42,43,44,45,46,47. Independent clinical studies reported successful cannulation of up to eight ducts per breast for local delivery of chemotherapy40,48,49. Visualizing full filling when delivering other solutions aimed at prevention or geared toward treatment would be worthwhile for similar reasons. The knowledge that the solution has reached all branches and terminal ends of the tree will be informative in assessing successful prevention or treatment.
We are not aware of any other intraductal imaging methods in mice33,34 or other animal models47 that afford the high resolution of TaOx nanoparticles. Of note, TaOx in the murine ductal tree outperforms FDA-approved contrast agents for diagnostic ductography3,16. As we continue to assess the ID ablative procedure for its ability to prevent breast cancer, we will be able to determine more precisely from which glands cancer arises with the help of added data given through imaging after ID delivery. For instance, one could determine whether a gland that was only partially filled is more likely than a non-injected gland to result in tumor formation, which addresses the safety profile and concern of unsuccessful injections on a high-risk woman. This technique has some limitations. This is a relatively challenging mouse technique that requires dexterity and proficiency of the operator to manipulate and successfully cannulate each duct. Each individual injection is an independent event thus unsuccessful injection on one or more glands may compromise result interpretation. Given the size of the murine mammary gland and fragility of the nipple, fluoroscopy or similar image-guidance technique is not available to inform in real-time when to stop infusion. This real time image-guidance will be a requirement for clinical implementation of local delivery of an ablative solution.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported, in part, by the National Cancer Institute R21 CA226579 and R01 CA258314 grants to LFS and by the National Institute of Biomedical Imaging and Bioengineering R01 EB029418 grant to EMS. We would like to thank the MSU Institute for Quantitative (IQ) Health Science and Engineering Imaging Core facility for use of their imaging systems and technical expertise. We would like to thank Dr. Danielle Ferguson for reviewing contents of the video and the figures for adherence to animal welfare guidelines.
Materials
| AnalyzeDirect v12.0 | Caliper | n/a | For micro-CT image processing |
| Carprieve, Carprofen 50 mg/mL | Allivet | 50647 | For anti-inflammatory treatment |
| Evans blue | Sigma | E2129-50G | For injection visualization |
| Hot water bath | Toolots | Yidu_HH-S2 | For preparing carprofen cups |
| Imaris | Bitplane | n/a | For confocal image processing |
| MediGel Sucralose Cups | ClearH2O | 74-02-5022 | For delivery of carprofen |
| Model 1705 RN Syringe, 50μL | Hamilton | 7655-01 | For intraductal injection |
| Photoshop 2021 | Adobe | n/a | For image processing |
| Quantum GX2 microCT Imaging System | Perkin Elmer | CLS149276 | For micro-CT image acquisition |
| Small Hub RN Needle, 34 gauge, custom (12° bevel angle, 0.375 in, point style 4) | Hamilton | 207434 | For intraductal injection |
| Stereo Microscope SZM Series | AmScope | SM-4TPZ-144 | For intraductal injection |
| Sterile blue food dye | McCormick | 930641 | For injection visualization |
| Sterile phosphate buffered saline (PBS) | ThermoFisher | 14190250 | For solution preparation |
| Stickers | DOT Scientific | DOTSCI-C50 | For preparing carprofen cups |
| Sucrose | Calbiochem | 8550-5KG | For intraductal injection |
| Syringes | Fisher | 14-826-79 | For preparing carprofen cups |
| Vortex | VWR | 10153-834 | For preparing carprofen cups |
| Warming pump/pad(s) | Braintree Scientific | HTP-1500 120V; AP-R 26E | For intraductal injection/preoperative preparation |
References
- Britt, K. L., Cuzick, J., Phillips, K. A. Key steps for effective breast cancer prevention. Nature Reviews Cancer. 20 (8), 417-436 (2020).
- Padamsee, T. J., Wills, C. E., Yee, L. D., Paskett, E. D. Decision making for breast cancer prevention among women at elevated risk. Breast Cancer Research. 19 (1), 34 (2017).
- Kenyon, E., et al. Ductal tree ablation by local delivery of ethanol prevents tumor formation in an aggressive mouse model of breast cancer. Breast Cancer Research. 21 (1), 129 (2019).
- Kuang, M., et al. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 253 (2), 552-561 (2009).
- Ansari, D., Andersson, R. Radiofrequency ablation or percutaneous ethanol injection for the treatment of liver tumors. World Journal Gastroenterol. 18 (10), 1003-1008 (2012).
- Zhang, W. Y., Li, Z. S., Jin, Z. D. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World Journal Gastroenterol. 19 (22), 3397-3403 (2013).
- Chin, M., Chen, C. L., Chang, K., Lee, J., Samarasena, J. Ethanol ablation of a peripheral nerve sheath tumor presenting as a small bowel obstruction. ACG Case Reports Journal. 3 (1), 31-32 (2015).
- Gueng, M. -. K., Chou, Y. -. H., Tiu, C. -. M., Chiou, S. -. Y., Cheng, Y. -. F. Pseudoaneurysm of the Breast Treated with Percutaneous Ethanol Injection. Journal of Medical Ultrasound. 22 (2), 114-116 (2014).
- Zhang, J., et al. Comparison between absolute ethanol and bleomycin for the treatment of venous malformation in children. Experimental and Therapeutics Medicine. 6 (2), 305-309 (2013).
- Wohlgemuth, W. A., et al. Ethanolgel sclerotherapy of venous malformations improves health-related quality-of-life in adults and children – results of a prospective study. European Radiology. 27 (6), 2482-2488 (2017).
- Steiner, F., FitzJohn, T., Tan, S. T. Ethanol sclerotherapy for venous malformation. ANZ Journal of Surgery. 86 (10), 790-795 (2016).
- Sannier, K., et al. A new sclerosing agent in the treatment of venous malformations. Study on 23 cases. Interventional Neuroradiology. 10 (2), 113-127 (2004).
- Dompmartin, A., et al. Radio-opaque ethylcellulose-ethanol is a safe and efficient sclerosing agent for venous malformations. European Radiology. 21 (12), 2647-2656 (2011).
- Slawson, S. H., Johnson, B. A. Ductography: how to and what if. Radiographics. 21 (1), 133-150 (2001).
- Sheiman, L. S., Levesque, P. H. The in’s and out’s of ductography: A comprehensive review. Current Problems in Diagnostic Radiology. 45 (1), 61-70 (2016).
- Chakravarty, S., et al. Tantalum oxide nanoparticles as versatile contrast agents for X-ray computed tomography. Nanoscale. 12 (14), 7720-7734 (2020).
- Krause, S., Brock, A., Ingber, D. E. Intraductal injection for localized drug delivery to the mouse mammary gland. Journal of Visualized Experiments: JoVE. (80), e50692 (2013).
- King, B. L., Love, S. M. The intraductal approach to the breast: raison d’etre. Breast Cancer Research. 8 (2), 206 (2006).
- Love, S. M., Barsky, S. H. Anatomy of the nipple and breast ducts revisited. Cancer. 101 (9), 1947-1957 (2004).
- Morhard, R., et al. Development of enhanced ethanol ablation as an alternative to surgery in treatment of superficial solid tumors. Scientific Reports. 7 (1), 8750 (2017).
- Morhard, R., et al. Understanding factors governing distribution volume of ethyl cellulose-ethanol to optimize ablative therapy in the liver. IEEE Transactions of Biomedical Engineering. 67 (8), 2337-2348 (2020).
- Hinck, L., Silberstein, G. B. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Research. 7 (6), 245-251 (2005).
- Paine, I. S., Lewis, M. T. The terminal end bud: The little engine that could. Journal of Mammary Gland Biology and Neoplasia. 22 (2), 93-108 (2017).
- Sivaraman, L., et al. Effect of selective ablation of proliferating mammary epithelial cells on MNU induced rat mammary tumorigenesis. Breast Cancer Res Treat. 73 (1), 75-83 (2002).
- Cardiff, R. D., Wellings, S. R. The comparative pathology of human and mouse mammary glands. Journal of Mammary Gland Biology and Neoplasia. 4 (1), 105-122 (1999).
- Arora, R., et al. Insights from imaging the implanting embryo and the uterine environment in three dimensions. Development. 143 (24), 4749-4754 (2016).
- Brock, A., et al. Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice. Science Translational Medicine. 6 (217), (2014).
- de Groot, J. S., et al. Intraductal cisplatin treatment in a BRCA-associated breast cancer mouse model attenuates tumor development but leads to systemic tumors in aged female mice. Oncotarget. 8 (37), 60750-60763 (2017).
- Wang, G., et al. Intraductal fulvestrant for therapy of ERalpha-positive Ductal Carcinoma in Situ (DCIS) of the breast- A preclinical study. Carcinogenesis. 40 (7), 903-913 (2019).
- Yoshida, T., et al. Effective treatment of ductal carcinoma in situ with a HER-2- targeted alpha-particle emitting radionuclide in a preclinical model of human breast cancer. Oncotarget. 7 (22), 33306-33315 (2016).
- Chun, Y. S., et al. Intraductally administered pegylated liposomal doxorubicin reduces mammary stem cell function in the mammary gland but in the long term, induces malignant tumors. Breast Cancer Research and Treatment. 135 (1), 201-208 (2012).
- Murata, S., et al. Ductal access for prevention and therapy of mammary tumors. Recherche en cancérologie. 66 (2), 638-645 (2006).
- Markiewicz, E., et al. High resolution 3D MRI of mouse mammary glands with intra-ductal injection of contrast media. Magnetic Resonance Imaging. 33 (1), 161-165 (2015).
- Markiewicz, E., et al. MRI ductography of contrast agent distribution and leakage in normal mouse mammary ducts and ducts with in situ cancer. Magnetic Resonance Imaging. 40, 48-52 (2017).
- Annunziato, S., et al. Comparative oncogenomics identifies combinations of driver genes and drug targets in BRCA1-mutated breast cancer. Nature Communications. 10 (1), 397 (2019).
- Rutkowski, M. R., et al. Initiation of metastatic breast carcinoma by targeting of the ductal epithelium with adenovirus-cre: a novel transgenic mouse model of breast cancer. Journal of Visualized Experiments: JoVE. (85), e51171 (2014).
- Xiang, D., Tao, L., Li, Z. Modeling breast cancer via an intraductal injection of cre-expressing adenovirus into the mouse mammary gland. Journal of Visualized Experiments: JoVE. (148), e59502 (2019).
- Barham, W., Sherrill, T., Connelly, L., Blackwell, T. S., Yull, F. E. Intraductal injection of LPS as a mouse model of mastitis: signaling visualized via an NF-kappaB reporter transgenic. Journal of Visualized Experiments: JoVE. (67), e4030 (2012).
- Chun, Y. S., et al. Intraductal administration of a polymeric nanoparticle formulation of curcumin (NanoCurc) significantly attenuates incidence of mammary tumors in a rodent chemical carcinogenesis model: Implications for breast cancer chemoprevention in at-risk populations. Carcinogenesis. 33 (11), 2242-2249 (2012).
- Stearns, V., et al. Preclinical and clinical evaluation of intraductally administered agents in early breast cancer. Science Translation Medicine. 3 (106), (2011).
- Okugawa, H., et al. Effect of perductal paclitaxel exposure on the development of MNU-induced mammary carcinoma in female S-D rats. Breast Cancer Research Treatment. 91 (1), 29-34 (2005).
- Falconer, I. R. The distribution of 131 I- or 125 I-labelled prolactin in rabbit mammary tissue after intravenous or intraductal injection. The Journal of Endocrinology. 53 (3), 58-59 (1972).
- Fiddler, T. J., Birkinshaw, M., Falconer, I. R. Effects of intraductal prolactin on some aspects of the ultrastructure and biochemistry of mammary tissue in the pseudopregnant rabbit. The Journal of Endocrinology. 49 (3), 459-469 (1971).
- Fiddler, T. J., Falconer, I. R. The effect of intraductal prolactin on protein and nucleic acid biosynthesis in the rabbit mammary gland. Biochemistry Journal. 115 (5), 58 (1969).
- Bourne, R. A., Bryant, J. A., Falconer, I. R. Stimulation of DNA synthesis by prolactin in rabbit mammary tissue. Journal of Cell Science. 14 (1), 105-111 (1974).
- Chadwick, A. Detection and assay of prolactin by the local lactogenic response in the rabbit. The Journal of Endocrinology. 27, 253-263 (1963).
- Clark, A., Bird, N. K., Brock, A. Intraductal delivery to the rabbit mammary gland. Journal Visualized Experiments: JoVE. (121), e55209 (2017).
- Mahoney, M. E., et al. Intraductal therapy of ductal carcinoma in situ: a presurgery study. Clinical Breast Cancer. 13 (4), 280-286 (2013).
- Love, S. M., et al. A feasibility study of the intraductal administration of chemotherapy. Cancer Prevention Research (Philadelphia). 6 (1), 51-58 (2013).

