Selection of Transporter-Targeted Inhibitory Nanobodies by Solid-Supported-Membrane (SSM)-Based Electrophysiology
Summary
Nanobodies are important tools in structural biology and pose a great potential for the development of therapies. However, the selection of nanobodies with inhibitory properties can be challenging. Here we demonstrate the use of solid-supported-membrane (SSM)-based electrophysiology for the classification of inhibitory and non-inhibitory nanobodies targeting electrogenic membrane transporters.
Abstract
Single domain antibodies (nanobodies) have been extensively used in mechanistic and structural studies of proteins and they pose an enormous potential as tools for developing clinical therapies, many of which depend on the inhibition of membrane proteins such as transporters. However, most of the methods used to determine the inhibition of transport activity are difficult to perform in high-throughput routines and depend on labeled substrates availability thereby complicating the screening of large nanobody libraries. Solid-supported membrane (SSM) electrophysiology is a high-throughput method, used for characterizing electrogenic transporters and measuring their transport kinetics and inhibition. Here we show the implementation of SSM-based electrophysiology to select inhibitory and non-inhibitory nanobodies targeting an electrogenic secondary transporter and to calculate nanobodies inhibitory constants. This technique may be especially useful for selecting inhibitory nanobodies targeting transporters for which labeled substrates are not available.
Introduction
Antibodies are composed of two identical heavy chains and two light chains that are responsible for the antigen binding. Camelids have heavy-chain only antibodies that exhibit similar affinity for their cognate antigen compared to conventional antibodies1,2. The single variable domain (VHH) of heavy-chain only antibodies retain the full antigen-binding potential and has been shown to be very stable1,2. These isolated VHH molecules or "nanobodies" have been implemented in studies related to membrane proteins biochemistry as tools for stabilizing conformations3,4, as inhibitors5,6, as stabilization agents7, and as gadgets for structure determination8,9,10. Nanobodies can be generated by the immunization of camelids for the pre-enrichment of B-cells that encode target-specific nanobodies and subsequent isolation of B cells, followed by cloning of the nanobody library and selection by phage display11,12,13. An alternate way to generate nanobodies is based on in vitro selection methods that rely on the construction of libraries and selection by phage display, ribosome display, or yeast display14,15,16,17,18,19,20. These in vitro methods require large library sizes but benefit from avoiding animal immunization and favor the selection of nanobodies targeting proteins with relatively low stability.
The small size of nanobodies, their high stability and solubility, strong antigen affinity, low immunogenicity, and relatively easy production, make them strong candidates for the development of therapeutics21,22,23. In particular, nanobodies inhibiting the activity of multiple membrane proteins are potential assets for clinical applications5,24,25,26. In the case of membrane transporters, to evaluate whether a nanobody has inhibitory activity, it is necessary to develop an assay that allows the detection of transported substrates and/or co-substrates. Such assays usually involve labeled molecules or the design of substrate-specific detection methods, which may lack a universal application. Furthermore, the identification of inhibitory nanobodies generally requires the screening of large numbers of binders. Thus, a method that can be used in a high-throughput mode and that does not rely on labeled substrates is essential for this selection.
SSM-based electrophysiology is an extremely sensitive, highly time-resolved technique that allows the detection of movement of charges across membranes (e.g., ion binding/transport)27,28. This technique has been applied to characterize electrogenic transporters, which are difficult to study using other electrophysiology techniques due to the relative low turnover of these proteins29,30,31,32,33,34,35. SSM electrophysiology does not require the use of labeled substrates, it is suitable for high-throughput screening, and either proteoliposomes or membrane vesicles containing the transporter of interest can be used. Here, we demonstrate that SSM-based electrophysiology can be used to classify transporter-targeted nanobodies with inhibitory and non-inhibitory properties. As a proof-of-principle, we describe the reconstitution of a bacterial choline transporter into liposomes, followed by detailed steps for immobilization of proteoliposomes on the SSM sensors. We next describe how to perform SSM-based electrophysiology measurements of choline transport and how to determine the half-maximal effective concentration (EC50). We then show how to use SSM-based electrophysiology to screen multiple nanobodies and to identify inhibitors of choline transport. Finally, we describe how to determine the half maximal inhibitory concentrations (IC50) of selected inhibitory nanobodies.
Protocol
1. Membrane protein reconstitution
- Mix 3 mL of E. coli polar lipids with 1 mL of phosphatidylcholine in a round bottom flask under a ventilated hood.
- Dry the lipid mixture for 20 min under vacuum using a rotary evaporator and a water bath at 37 °C to remove chloroform. If needed, dry further under nitrogen or argon gas.
- Using TS buffer (20 mM Tris-HCl pH 8.0, 150 mM NaCl) containing 2 mM β-mercaptoethanol, resuspend lipids to 25 mg/mL.
- Aliquot lipids in 500 µL aliquots, flash freeze in liquid nitrogen, and store at -80 °C.
- Thaw one 500 µL aliquot of lipids and dilute 1:1 using TS buffer containing 2 mM β-mercaptoethanol.
- Extrude the lipid suspension 15 times using a 400 nm membrane.
- Dilute the lipid suspension to have a final lipid concentration of 4.4 mg/mL.
- Add n-dodecyl-β-D-maltoside (DDM) to have a final concentration of 0.2% and leave it rotating for 1 h at 200 rpm at room temperature (RT).
- Add the purified protein to the lipids using a lipid-to-protein ratio between 1:10 and 1:100 (w:w).
NOTE: The ratio needs to be adjusted depending on the strength of the signal detected in SSM-electrophysiology measurements of substrate transport (see below). To obtain larger signals, use smaller lipid-to-protein ratios. - Incubate the mixture rotating at 200 rpm for 1 h at RT.
- Add 30 mg/mL of polystyrene adsorbent beads, pre-washed in TS buffer.
NOTE: Add polystyrene beads stepwise. - Incubate the beads-lipids mixture for 30 min at RT under slow stirring.
- To remove the beads, let the beads-lipids mixture stand so that the beads settle down. Transfer the solution to a new tube and leave the beads behind. Add 30 mg/mL of fresh polystyrene adsorbent beads to the separated lipid mixture.
- Incubate the mixture for 1 h at 4 °C under slow stirring.
- Separate the beads from the mixture as described in step 1.13 and add 30 mg/mL of fresh polystyrene adsorbent beads.
- Incubate the mixture for 16 h at 4 °C.
- Separate the beads from the mixture as described in step 13 and add 30 mg/mL of fresh polystyrene adsorbent beads.
- Incubate the mixture for 2 h at 4 °C for a fourth and final wash.
- Centrifuge at 110,000 x g for 30 min at 4 °C.
- Wash the pellet with 500 µL of TS buffer containing 2 mM β-mercaptoethanol.
- Centrifuge again at 110,000 x g for 30 min at 4 °C.
- Resuspend the pellet to a final lipid concentration of 25 mg/mL in TS buffer with 2 mM β-mercaptoethanol.
- Estimate the protein concentration using an in-gel or amido black assay36.
- Aliquot the proteoliposomes, flash freeze in liquid nitrogen, and store at -80 °C.
2. Chip preparation
- Fill a single sensor chip with 50-100 µL of 0.5 mM 1-octadecanethiol solution (resuspended in isopropanol).
- Incubate the chip with the solution for 30 min at RT.
- Remove the thiol solution by tapping the chip on a tissue.
- Rinse the sensor 3 times with 5 mL of pure isopropanol.
- Rinse the sensor 3 times with 5 mL of double distilled water.
- Dry the sensor by tapping on a tissue paper.
- Apply 1.5 µL of 7.5 µg/µL 1,2-diphytanoyl-sn-glycero-3-phosphocholine (lipids dried in a rotatory evaporator and resuspended in n-decane).
- Immediately after, fill the sensor with 50 µL of non-activating SSM buffer, which does not contain the substrate. This will lead to a spontaneous formation of the SSM layer.
NOTE: The SSM buffer should be optimized beforehand to determine optimal conditions that have low background noise. A general buffer containing 30 mM HEPES pH 7.4, 5 mM MgCl2, 140 mM NaCl, can be used as a starting point. The SSM buffer without the substrate is used for washing before and after the measurement (non-activating buffer). To avoid buffer mismatch, use the non-activating buffer to prepare the buffer containing the substrate (activating buffer). The substrate can be added either directly as a powder or in a small volume from a high concentration stock to avoid dilutions. - Thaw proteoliposomes from step 1.24 at RT.
- Dilute proteoliposomes between 1:5 and 1:100 (proteoliposomes:buffer, (v:v)) in the non-activating SSM buffer (here 1:20).
- Sonicate proteoliposomes for 20-30 s or 3 times for 10 s, placing on ice in between sonication, if necessary. Here a water bath sonicator at 45 kHz was used.
- Apply 5-10 µL of the diluted sonicated proteoliposomes sample on the surface of the sensor without touching it.
- Centrifuge the chips with the solution at RT for 30 min using a speed between 2,000 and 3,000 x g.
NOTE: Use 50 mL tubes with a flat bottom. Carefully place the sensor chips upright using tweezers. 6-well plates and a centrifuge with a plate holder can also be used. - Use the sensor chips on the same day.
3. Measuring the solute transportation: determination of saturation conditions
NOTE: As proof-of-principle, these experiments were performed using a bacterial choline transporter reconstituted in liposomes following the protocol described above. The step-by-step process of determining saturating conditions of the substrate choline prior to the measurement of inhibition by nanobodies is shown here.
- Prepare 1-2 L of the non-activating SSM buffer.
NOTE: Prepare and use the same SSM buffer stock for all activating and non-activating buffers throughout all measurements. - Take 10 clean tubes and transfer 10 mL of the non-activating SSM buffer into each.
- Add the substrate into the tubes from step 3.2 using a series of concentrations around the expected half maximum concentration (here 15, 10, 5, 1, 0.5, 0.1, 0.05, 0.01, 0.005, 0.001 mM of choline) to prepare the activating SSM buffers. Use a high concentration stock to avoid dilutions.
- Switch on the SSM machine.
- Start the SSM software and let the machine initialize automatically. Set the saving path for data and confirm by hitting the OK button. Select the standard Initial Cleaning Protocol in the workflow options and click Run.
- Mount the proteoliposome coated chip on the socket, move the arm to lock the chip, and close the mounted chip with the cap.
- Select the program CapCom in the workflow and let it Run to determine the conductivity and capacitance. Confirm that the conductivity is below 5 nS and the capacitance is between 15 and 35 nF before using it for the measurement.
NOTE: A capacitance value of 15-35 nF and conductance below 5 nS are recommended by the manufacturer when using a 3 mm chip. - Transfer the activating solutions into vials and position the buffers in the probe sampler.
- Transfer the non-activating buffer into a reservoir and position it next to the chip holder at the reservoir position on the right.
- Create a protocol for the workflow using a sequence of non-activating (B), activating (A), and non-activating (B) solutions (B-A-B sequence) and a loop that performs three measurements and moves to the next activating buffer for all 10 buffers prepared in step 3.3. Use the default flow rate at 200 µL/s using 1 s – 1 s – 1 s flow times for the B-A-B sequence. Click Play to start the measurement.
NOTE: A typical experiment consists of the sequential flow of non-activating (B), activating (A), and non-activating (B) solutions (written as B-A-B; see Figure 1A). The immobilized proteoliposomes on the sensor will be washed with the solutions. Therefore, the B-A solution exchange generates a substrate concentration gradient, which drives the electrogenic transport reaction. - Save the protocol and let the workflow run by clicking on the Play button. Perform the same type of experiment but using protein-free liposomes. This is highly important as it would show the intensity of background currents. This should be considered when analyzing data of electrogenic transport measured with proteoliposomes (Figure 1C).
- Use any preferred software for data analysis to plot the measured current versus time. Read out the peak current manually, or if using the software, use the function for peak height estimation in the range of the addition of the activating buffer.
- Plot the peak current against the substrate concentration to determine the EC50 of the substrate via the nonlinear regression (Figure 1B,C). Read out the lowest concentration at which the peak current reaches a maximum value, this concentration corresponds to saturating conditions.
NOTE: It is important to consider that the number of proteoliposomes that remain immobilized varies from chip-to-chip. This variation is evident as the peak currents at identical measurement conditions will show different amplitudes. Therefore, it is necessary to normalize the current amplitudes of measurements performed on each chip separately before comparing measurements among different chip preparations.
4. Serial classification of inhibitory and non-inhibitory nanobodies
NOTE: This section shows how to measure choline transport in the presence of nanobodies that bind specifically to the bacterial choline transporter. Smaller peak currents in the presence of nanobodies indicate transport inhibition. Non-inhibitory nanobodies will not impact substrate transport, i.e., no decrease of the peak current signal.
- Prepare 1-2 L of non-activating SSM buffer.
- Transfer 50 mL of the non-activating SSM buffer into a clean tube. Add the substrate choline to a final concentration of 5 mM (saturating conditions). Use this for a positive control measurement.
- Transfer 10 mL of the non-activating SSM buffer into a clean tube. Add the substrate choline to a final concentration of 5 mM (saturating conditions) and add nanobody to a final concentration of 500 nM.
- Repeat step 4.3 for each nanobody to prepare the activating solutions.
NOTE: If the purified nanobodies are resuspended in a different buffer than the SSM buffer, their addition to the activating and non-activating buffers will lead to a buffer mismatch. A buffer mismatch should be avoided since it can lead to high noise. Exchanging the buffer of the purified nanobodies with the SSM buffer can help to avoid this issue. Furthermore, using nanobody concentrations that allow to reach saturating conditions is recommended. Considering that the binding constants of nanobodies are generally below 100 nM, a nanobody concentration of 500 nM is recommended for this experiment. However, it is important to pre-screen for optimal concentrations. - Start the SSM machine and measure the capacitance and conductivity of the proteoliposome coated chip as described in steps 3.4-3.7.
- Transfer the activating solution without a nanobody into a vial and place the buffer in the probe sampler. Transfer the non-activating buffer without a nanobody into a reservoir and position it in the probe sampler.
- Transfer the activating solutions containing nanobodies into vials and position the buffers in the probe sampler. Transfer the non-activating buffers containing nanobodies into vials and position the buffers in the probe sampler.
- Create a protocol for the workflow using a sequence of non-activating (B), activating (A), and non-activating (B) solutions (B-A-B sequence).
- Create a loop that performs the following: three measurements of the B-A-B sequence using buffers without nanobody, two measurements of the B-A-B sequence with buffers containing a nanobody, 120 s delay time for incubation with the nanobody, then 3 measurements of the B-A-B sequence with buffers containing the nanobody.
- Save the workflow and let it run by clicking the Play button.
NOTE: This workflow will measure the initial conditions of the transport without inhibition by running the B-A-B protocol 3 times using non-activating (B) and activating buffers (A) without the nanobody (Figure 1B,C), followed by the measurement of the nanobody effect on the transport by running the B-A-B protocol 5 times with the non-activating and activating buffers containing nanobodies (Figure 2A). The second order binding kinetics dictate the interaction of nanobodies and their target proteins. Therefore, it is important to use a time delay in the B-A step in order to give enough time for nanobodies to bind to transporters in proteoliposomes on the chip. Optimal times depend on the nanobody concentration i.e., at lower concentrations longer times are required. The first two measurements are required to adapt the system to the new conditions and the second run should be performed after a delay time of 120 s. Only the following three measurements should be used for data analysis. - Create a new protocol for the workflow using a sequence of non-activating (B), activating (A), and non-activating (B) solutions (B-A-B sequence) and a loop of 5 measurements to wash out the reversibly bound nanobody.
NOTE: Optionally, include an incubation step to allow dissociation of nanobodies with slow kinetics. - Save the workflow and let it run by clicking the Play button.
- Compare the last peak current of the measurements with the initial substrate-only measurement in step 4.10. The nanobody has been successfully washed out and the initial conditions have been reestablished if the peak current reaches the initial value, otherwise repeat steps 4.12-4.13 or change to a new chip.
- Repeat steps 4.6-4.13 and use individual chips for each nanobody screen (Figure 2C) or repeat with multiple nanobodies using the same chip (Figure 2D).
- Use any preferred software for data analysis to plot the measured current versus time. Read out the peak current manually, or if available, in the used software, automatically select the function for peak height estimation in the range of the addition of the activation buffer.
- Normalize the peak current in presence of the nanobody, based on the preceding substrate-only measurement. Plot the peak currents in a histogram and compare the peak currents of the substrate only measurements to the peak currents measured in the presence of nanobodies (Figure 2C,D) to identify inhibitory nanobodies.
NOTE: Normalization of the determined peak currents of each individual run with a nanobody should be performed considering the peak current in the absence of the nanobody from the preceding measurement. Also, since the number of proteoliposomes that remain immobilized vary from chip-to-chip, it is important to normalize the current amplitudes of measurements performed on each chip separately before comparing measurements among different chip preparations.
5. IC50 measurement with inhibitory nanobodies
NOTE: After identifying inhibitory nanobodies, it is possible to determine their half maximal inhibitory concentration (IC50). This is done by measuring the transport of choline at constant concentration, while varying concentrations of the inhibitory nanobody.
- Prepare 1-2 L of the non-activating SSM buffer.
- Transfer 50 mL of the non-activating SSM buffer into a clean tube. Add the substrate choline to a final concentration of 5 mM (saturating conditions). Use this as the activating solution for positive control.
- Take 8 clean tubes and add 5 mL of the non-activating solution into each. Add the substrate choline to a final concentration of 5 mM (saturating conditions). Add the inhibitory nanobody to the tubes at concentrations in the expected IC50 range (here 500 nM – 1 nM).
- Take 8 clean tubes and add 10 mL of the non-activating solution into each. Add inhibitory nanobody to each tube individually at the same concentration as in step 5.3. This corresponds to the non-activating buffer.
NOTE: This will generate a series of activating and non-activating buffer pairs at different concentrations of the same inhibitory nanobody. - Start the SSM setup and measure the capacitance and conductivity of the proteoliposome coated chip as described in steps 3.4-3.7.
- Transfer the activating solution without nanobody into a vial and place it in the probe sampler. Transfer the non-activating buffer without nanobody into a reservoir and position it at the reservoir position next to the chip holder on the right.
- Transfer the activating solutions containing nanobodies into vials and position the buffers in the probe sampler. Transfer the non-activating buffers containing nanobodies into vials and position the buffers in the probe sampler.
- Create a protocol for the workflow using a sequence of non-activating (B), activating (A), and non-activating (B) solutions (B-A-B sequence). Include a loop to measure each concentration 2 times, incubate for 120 s and measure 3 more times. The workflow will start with the positive control of substrate-only followed by the lowest concentration of the nanobody. Each nanobody measurement will be followed by a subsequent measurement of the positive control with substrate-only to restore the initial peak amplitude, before moving to the next higher nanobody concentration.
NOTE: The second order kinetics dictate the binding of nanobodies. Therefore, it is important to use a time delay in the B-A step. Optimal times depend on the nanobody concentration i.e., at lower concentrations longer times are required; here 120 s was used with satisfactory results. - Use any preferred software for data analysis to plot the measured current versus time. Read out the peak current manually, or if using software, select the function for the peak height estimation in the range of the addition of the activation buffer (Figure 3A). Plot the peak currents against the nanobody concentration to determine the IC50 via non-linear regression (Figure 3B).
NOTE: Normalize the current amplitudes of measurements performed for each individual chip before comparing measurements among different chip preparations.
6. Cleaning of sensors
- Rinse the single sensor chips after use with 10 mL of distilled water.
- Dry the chip by tapping it on a tissue paper.
- Fill the sensor cavity of the chip with 100 µL of pure isopropanol and incubate for 10 min at RT.
- Place a cotton swab in pure isopropanol and incubate for 1-3 min.
- Use the presoaked cotton swabs and gently rotate on the sensor's surface without pressure to remove residues.
- Rinse the sensor with 5 mL of pure isopropanol.
- Rinse the sensor with 10 mL of distilled water.
- Dry the sensor by tapping the chip on a tissue.
- Let the sensor dry overnight at RT, and store at RT under dry conditions.
NOTE: Sensors can be re-used up to 4-5 times when cleaned and stored properly.
Representative Results
SSM-based electrophysiology has been extensively used for the characterization of electrogenic transporters. In the protocol presented here, we show how to use SSM-based electrophysiology to classify nanobodies targeting a secondary transporter (here a bacterial choline symporter) based on their inhibitory and non-inhibitory properties. One of the most useful features of this technique is that it allows for the high-throughput screening of multiple buffer conditions. This particular characteristic is beneficial for the analysis of nanobody libraries, which after the selection of binders can be constituted from a few to dozens of nanobodies. In a standard experiment, a stable lipid monolayer is assembled on a sensor chip. After applying the proteoliposomes preparation containing the choline transporter, a check for good conductivity and capacitance is performed as this is essential for the success of the experiment. In case that the integrity of the membrane is compromised during an experiment, which is easily observed due to the high noise background currents, changing to a new chip is recommended as recovering low noise conditions is rather difficult. In general, we have observed very good reproducibility among measurements of transport and inhibition by nanobodies when using different chips.
To decide about the substrate concentration to be used during a screening of nanobodies, electrogenic transport was first measured under different substrate concentrations to determine EC50 (Figure 1B,C). A substrate concentration that corresponds to saturating conditions was selected (Figure 1C). This substrate concentration was then kept constant in all activating buffers. For this particular example, we selected 5 mM choline.
For the screening of nanobodies, the nanobody must be added to both non-activating and activating buffers. When nanobodies were added to only the activating buffer, it was not possible to observe inhibition of the electrogenic transport. We speculate that this is due to an incomplete occupation of all nanobody binding sites in the transporter population on the chip, thereby revealing the importance of pre-incubation with nanobodies in non-activating conditions. To ensure that all sites are likely to be occupied, a time delay step was included during the application of the first non-activating buffer step to allow the saturation of nanobody binding sites on the transporter population. Incubation times ranging from 2-60 min have been tested with reproducible results. Keep in mind that optimal times of incubation depend on the nature of the nanobody binder and its concentration during the experiment (as well as the concentration of transporter in proteoliposomes on the chip). Therefore, it is recommended to try different incubation times. In any case, as a rule of thumb, the lower the nanobody concentration, the longer the incubation time required. We tested incubation times of 2 min, 20 min, 30 min, and 60 min for different nanobodies but did not detect further transport inhibition.
The effect of inhibitory nanobodies on electrogenic transport is visualized from the decrease of peak currents amplitudes (Figure 2A,C,D). Non-inhibitory nanobodies, on the other hand, do not affect peak currents. After running the washing protocol to allow nanobodies unbinding, a recovery of 80 to 95% of the initial peak current amplitude was observed (Figure 2A,C,D). We have performed a similar experiment but in the presence of liposomes without the transporter protein. When changing from non-activating to activating conditions, no significant artifact currents was introduced by nanobodies present in these buffers (Figure 2B). Running this control experiment is recommended as it is important to know whether changes in peak currents arise from artifacts or not.
After the selection of nanobodies with inhibitory properties, we determined IC50 values for individual nanobodies (Figure 3A,B). For this particular experiment, it is recommended to start with a low concentration of nanobodies and then move towards high concentration during the assay. The calculation of the inhibition for each concentration was then performed by comparing peak currents measured before and after the application of nanobody. To avoid unspecific binding of nanobodies to surfaces, which can be particularly problematic when using low nanobody concentrations, it is advised to follow a similar protocol to that described by Kermani et al.37, where 50 µg/mL of bovine serum albumin was added to the buffers, preventing this deleterious effect. Adding detergents such as Tween or Triton for this purpose should be avoided as these would dissolve lipid membranes.
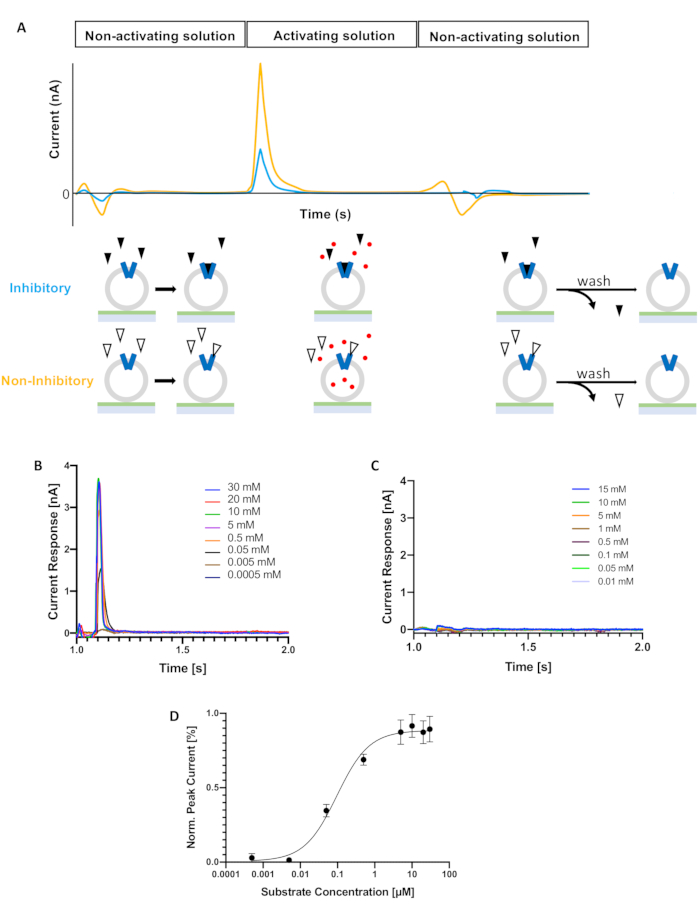
Figure 1: SSM-based electrophysiology. (A) Protocol for transient currents measurement. A non-activating solution is replaced by an activating solution followed by the flow of non-activating solution to restore initial conditions. During the first step, nanobodies bind to the transporter. When switching to the activating solution, the substrate gradient drives the electrogenic transport (orange curve). In the presence of an inhibitory nanobody, the peak current shows a smaller amplitude (blue curve). After finishing the protocol and running solutions without nanobody (wash), unbinding of nanobodies occurs. In the schematic, proteoliposomes with reconstituted protein (blue) are immobilized on the SSM sensor. Triangles and red circles represent nanobodies and substrate, respectively. (B) Electrogenic choline transport in the absence of nanobodies. Peak currents measured during activating conditions are shown for different substrate concentrations. (C) Representative measurement of currents during activating conditions in the absence of transporter protein at different substrate concentrations. (D) Plot of substrate concentration versus peak currents amplitude. The EC50 determined was 95 ± 11 µM choline. Error bars indicate standard deviation (n=3 biological replicates, n=3 technical replicates). Please click here to view a larger version of this figure.
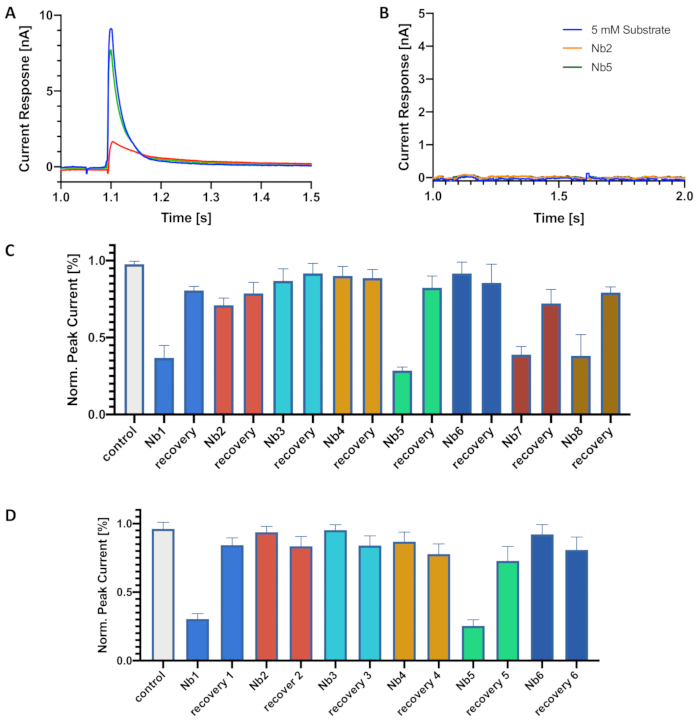
Figure 2: Screening and classification of inhibitory and non-inhibitory nanobodies. (A) Electrogenic choline transport in the presence of a nanobody. Peak currents measured during activating conditions are shown in the absence of nanobody (blue), in the presence of an inhibitory nanobody (red), and after nanobody unbinding (green). (B) Measurement of currents during activating conditions in the absence of transporter protein. Traces show recordings in the absence of nanobody (blue), in the presence of an inhibitory nanobody (green), and in the presence of a non-inhibitory nanobody (red). (C,D). Histograms showing peak currents measured during activating conditions in the presence of nanobodies and after nanobody unbinding (recovery). Panel C shows the results of measurements using individual chips per nanobody. Panel D shows the results from a serial measurement using one chip. Nanobodies are indicated as Nb. Error bars indicate the standard deviation (n=3 biological replicates, n=2 technical replicates). Please click here to view a larger version of this figure.
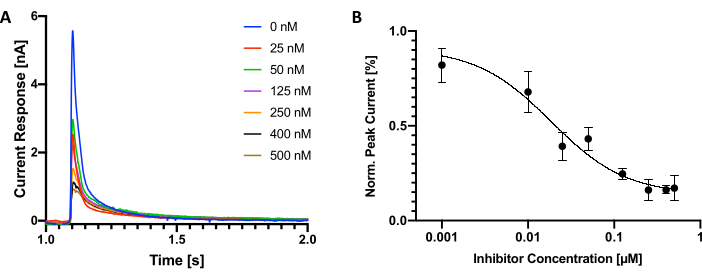
Figure 3: Determination of IC50 of an inhibitory nanobody. (A) Electrogenic choline transport and inhibition by a nanobody. Peak currents measured during activating conditions are shown for different nanobody concentrations. (B) Plot of peak currents amplitude vs nanobody concentration from a serial measurement with an inhibitory nanobody. The IC50 determined was 18 ± 2 nM. Error bars indicate the standard deviation (n=3 biological replicates, n=3 technical replicates). Please click here to view a larger version of this figure.
Discussion
The technique presented here classifies nanobodies with inhibitory and non-inhibitory properties targeting electrogenic transporters. Assessing the substrate transport is possible due to the detection of the movement of charges through the transporter embedded in the membrane of proteoliposomes. Some of the critical steps during the setup of an experiment are reconstitution of active protein in liposomes, preparation of stable monolayers on SSM chips, and recovering of initial conditions after the application of the wash protocol to remove bound nanobody molecules. Once the membrane protein is reconstituted at an appropriate lipid-to-protein ratio, a general SSM protocol can be established using the native substrate. It is crucial to perform control experiments using protein-free liposomes to reveal noise currents that would need to be subtracted from the currents measured using proteoliposomes. However, if noise currents are too large, we recommend trying protein reconstitution using different lipids, or screen for buffer conditions that minimize these deleterious signals. After successfully establishing conditions for an SSM assay, screening of nanobodies can be performed. A very useful option that may help in faster screening of nanobodies is to perform high-throughput assays using a single SSM sensor chip. This reduces the time of manipulation of chips and buffers and reduces the costs. However, because during this type of assay multiple nanobodies are applied sequentially, it is important to ensure that the applied nanobody can be washed away after the measurement. A stringent washing cycle may need to be implemented to unbind some nanobodies in case that reduced peak current amplitudes are detected in the absence of nanobody. We recommend using, as the starting point, the washing conditions described here. If increasing the washing volume or the number of cycles does not help, individual chips would need to be used to screen each nanobody separately. In all the cases examined here, the binding of nanobodies was reversible and a high-throughput protocol could be applied. In our experimental setup, we could not recover the full amplitude of the initial peak current after measurements with nanobodies (Figure 1A; Figure 2C,D). However, in most cases, the magnitude of the peak currents recovered ranged between 80 and 95% of the amplitude measured before applying nanobodies (Fig. 2C,D). We speculate that this could be a consequence of washing away a fraction of the proteoliposomes adhered to the chip, or due to slow kinetics of unbinding of some inhibitory nanobodies, or a combination of both. In either case, it was still possible to continue assaying further nanobodies as electrogenic transport was measurable. This is shown in Figure 2D, where we screened six nanobodies using a single chip preparation.
The high-throughput characteristic is one of the most significant advances of the method presented. In addition, in contrast to other approaches, this method allows for the selection of inhibitory nanobodies targeting electrogenic transporters for which labeled substrates are not available. A fast identification of inhibitory nanobodies can help to speed up the research aiming to identify novel applications of nanobodies as drugs. Their apparent advantages compared to similar therapies such as antibody treatments are numerous, starting with a smaller size which helps them propagate further into tissues or cells, to their low production costs and high stability.
SSM-based electrophysiology has been used in the past for the characterization of electrogenic transporters in membrane vesicles29,38. These types of experiments are advantageous as they do not depend on the protein purification and reconstitution protocols. We speculate that performing the selection of inhibitory nanobodies using membrane vesicles is feasible. This would help to reduce costs and avoid manipulations of purified proteins.
SSM-based electrophysiology is a strong technique to screen for nanobody inhibitors of multiple membrane proteins that exhibit electrogenic transport. We envision that SSM-based electrophysiology will become an important tool for the selection of inhibitory nanobodies and other antibodies with potential clinical applications.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Cedric A. J. Hutter and Markus A. Seeger from the Institute of Medical Microbiology at the University of Zurich, and Gonzalo Cebrero from Biozentrum of the University of Basel for collaboration in the generation of synthetic nanobodies (sybodies). We thank Maria Barthmes and Andre Bazzone from NANION Technologies for technical assistance. This work was supported by the Swiss National Science Foundation (SNSF) (PP00P3_170607 and NANION Research Grant Initiative to C.P.).
Materials
| 1-octadecanethiol solution | Sigma Aldrich | O1858-25ML | |
| 1,2-diphytanoyl-sn-glycero-3-phosphocholine | Avanti Polar Lipids | 850356C-25mg | |
| Bio-Beads SM-2 Adsorbent (Polystyrene adsorbent beads) | BioRad | #152-3920 | |
| PD 10 Desalting Columns | GE Healthcare | GE17-0851-01 | |
| Filter 200 nm membrane | Whatman Nucleopore | WHA800282 | |
| 2-Propanol | Merck | 33539-1L-R | |
| n-Decane | Sigma Aldrich | 8034051000 | |
| n-dodecyl-ß-D-maltoside (DDM) | Avanti Polar Lipids | 850520P-25g | |
| Sodium Chloride | AppliChem | 131659.1211 | |
| (SSM setup) SURFE2R N1 | Nanion | —– | |
| SURFE2R N1 Single Sensor Chips | Nanion | # 161001 | |
| Trizma Base | Sigma Aldrich | T1503 | |
| E. coli Polar Lipid Extract | Avanti Polar Lipids | 100600C | |
| Egg PC L-α-phosphatidylcholine | Avanti Polar Lipids | 840051C |
References
- Braden, B. C., Goldman, E. R., Mariuzza, R. A., Poljak, R. J. Anatomy an antibody molecule: structure, kinetics, thermodynamics, and mutational studies of the antilysozyme antibody D1.3. Immunology Reviews. 163, 45-57 (1998).
- Hamers-Casterman, C., et al. Naturally occurring antibodies devoid of light chains. Nature. 363, 446-448 (1993).
- Perez, C., et al. Structural basis of inhibition of lipid-linked oligosaccharide flippase PglK by a conformational nanobody. Science Reports. 7, 46641 (2017).
- Grahl, A., Abiko, L. A., Isogai, S., Sharpe, T., Grzesiek, S. A high-resolution description of beta1-adrenergic receptor functional dynamics and allosteric coupling from backbone NMR. Nature Communication. 11, 2216 (2020).
- Schenck, S., et al. Generation and characterization of anti-VGLUT nanobodies acting as inhibitors of transport. Biochimie. 56, 3962-3971 (2017).
- Mireku, S. A., Sauer, M. M., Glockshuber, R., Locher, K. P. Structural basis of nanobody-mediated blocking of BtuF, the cognate substrate-binding protein of the Escherichia coli vitamin B12 transporter BtuCD. Science Reports. 7, 14296 (2017).
- Manglik, A., Kobilka, B. K., Steyaert, J. Nanobodies to study G protein-coupled receptor structure and function. Annual Reviews of Pharmacology Toxicology. 57, 19-37 (2017).
- Rasmussen, S. G., et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 469 (2), 175-180 (2011).
- Jiang, X., et al. Crystal structure of a LacY-nanobody complex in a periplasmic-open conformation. Proceeding of the National Academy of Science U. S. A. 113, 12420-12425 (2016).
- Geertsma, E. R., et al. Structure of a prokaryotic fumarate transporter reveals the architecture of the SLC26 family. Nature Structural Molecular Biology. 22, 803-808 (2015).
- Harmsen, M. M., De Haard, H. J. Properties, production, and applications of camelid single-domain antibody fragments. Applied Microbiology and Biotechnology. 77, 13-22 (2007).
- Pardon, E., et al. A general protocol for the generation of nanobodies for structural biology. Nature Protocols. 9, 674-693 (2014).
- Nguyen, V. K., Desmyter, A., Muyldermans, S. Functional heavy-chain antibodies in Camelidae. Advances in Immunology. 79, 261-296 (2001).
- Zimmermann, I., et al. Synthetic single domain antibodies for the conformational trapping of membrane proteins. Elife. 7, 34317 (2018).
- McMahon, C., et al. Yeast surface display platform for rapid discovery of conformationally selective nanobodies. Nature Structural Molecular Biology. 25, 289-296 (2018).
- Olichon, A., de Marco, A. Preparation of a naive library of camelid single domain antibodies. Methods in Molecular Biology. 911, 65-78 (2012).
- Moutel, S., et al. NaLi-H1: A universal synthetic library of humanized nanobodies providing highly functional antibodies and intrabodies. Elife. 5, 16228 (2016).
- Yan, J., Li, G., Hu, Y., Ou, W., Wan, Y. Construction of a synthetic phage-displayed nanobody library with CDR3 regions randomized by trinucleotide cassettes for diagnostic applications. Journal of Translational Medicine. 12, 343 (2014).
- Sabir, J. S., et al. Construction of naive camelids VHH repertoire in phage display-based library. Comptes Rendus Biologies. 337, 244-249 (2014).
- Yau, K. Y., et al. Selection of hapten-specific single-domain antibodies from a non-immunized llama ribosome display library. Journal of Immunology Methods. 281, 161-175 (2003).
- vander Linden, R. H., et al. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochimica et Biophysica Acta. 1431, 37-46 (1999).
- Dumoulin, M., et al. Single-domain antibody fragments with high conformational stability. Protein Science. 11, 500-515 (2002).
- Iezzi, M. E., Policastro, L., Werbajh, S., Podhajcer, O., Canziani, G. A. Single-domain antibodies and the promise of modular targeting in cancer imaging and treatment. Frontiers in Immunology. 9, 273 (2018).
- Yu, X., et al. Nanobodies derived from camelids represent versatile biomolecules for biomedical applications. Biomaterials Science. 8, 3559-3573 (2020).
- Jahnichen, S., et al. CXCR4 nanobodies (VHH-based single variable domains) potently inhibit chemotaxis and HIV-1 replication and mobilize stem cells. Proceedings of the National Academy of Science U. S. A. 107, 20565-20570 (2010).
- Nguyen, V. S., et al. Inhibition of type VI secretion by an anti-TssM llama nanobody. PLoS One. 10, 0122187 (2015).
- Bazzone, A., Barthmes, M., Fendler, K. SSM-Based Electrophysiology for Transporter Research. Methods in Enzymology. 594, 31-83 (2017).
- Schulz, P., Garcia-Celma, J. J., Fendler, K. SSM-based electrophysiology. Methods. 46, 97-103 (2008).
- Barthmes, M., Liao, J., Jiang, Y., Bruggemann, A., Wahl-Schott, C. Electrophysiological characterization of the archaeal transporter NCX_Mj using solid supported membrane technology. Journal of General Physiology. 147, 485-496 (2016).
- Watzke, N., Diekert, K., Obrdlik, P. Electrophysiology of respiratory chain complexes and the ADP-ATP exchanger in native mitochondrial membranes. Biochimie. 49, 10308-10318 (2010).
- Zuber, D., et al. Kinetics of charge translocation in the passive downhill uptake mode of the Na+/H+ antiporter NhaA of Escherichia coli. Biochim Biophys Acta. 1709, 240-250 (2005).
- Garcia-Celma, J. J., Smirnova, I. N., Kaback, H. R., Fendler, K. Electrophysiological characterization of LacY. Proceedings of the National Academy of Science U. S. A. 106, 7373-7378 (2009).
- Bazzone, A., Madej, M. G., Kaback, H. R., Fendler, K. pH regulation of electrogenic sugar/H+ symport in MFS sugar permeases. PLoS One. 11, 0156392 (2016).
- Williamson, G., et al. A two-lane mechanism for selective biological ammonium transport. Elife. 9, 57183 (2020).
- Mirandela, G. D., Tamburrino, G., Hoskisson, P. A., Zachariae, U., Javelle, A. The lipid environment determines the activity of the Escherichia coli ammonium transporter AmtB. FASEB Journal. 33, 1989-1999 (2019).
- Kaplan, R. S., Pedersen, P. L. Determination of microgram quantities of protein in the presence of milligram levels of lipid with amido black 10B. Annals of Biochemistry. 150, 97-104 (1985).
- Kermani, A. A., et al. The structural basis of promiscuity in small multidrug resistance transporters. Nature Communication. 11, 6064 (2020).
- Weitz, D., et al. Functional and structural characterization of a prokaryotic peptide transporter with features similar to mammalian PEPT1. Journal of Biological Chemistry. 282, 2832-2839 (2007).

