Generation of 3D Whole Lung Organoids from Induced Pluripotent Stem Cells for Modeling Lung Developmental Biology and Disease
Summary
The article describes step wise directed differentiation of induced pluripotent stem cells to three-dimensional whole lung organoids containing both proximal and distal epithelial lung cells along with mesenchyme.
Abstract
Human lung development and disease has been difficult to study due to the lack of biologically relevant in vitro model systems. Human induced pluripotent stem cells (hiPSCs) can be differentiated stepwise into 3D multicellular lung organoids, made of both epithelial and mesenchymal cell populations. We recapitulate embryonic developmental cues by temporally introducing a variety of growth factors and small molecules to efficiently generate definitive endoderm, anterior foregut endoderm, and subsequently lung progenitor cells. These cells are then embedded in growth factor reduced (GFR)-basement membrane matrix medium, allowing them to spontaneously develop into 3D lung organoids in response to external growth factors. These whole lung organoids (WLO) undergo early lung developmental stages including branching morphogenesis and maturation after exposure to dexamethasone, cyclic AMP and isobutylxanthine. WLOs possess airway epithelial cells expressing the markers KRT5 (basal), SCGB3A2 (club) and MUC5AC (goblet) as well as alveolar epithelial cells expressing HOPX (alveolar type I) and SP-C (alveolar type II). Mesenchymal cells are also present, including smooth muscle actin (SMA), and platelet-derived growth factor receptor A (PDGFRα). iPSC derived WLOs can be maintained in 3D culture conditions for many months and can be sorted for surface markers to purify a specific cell population. iPSC derived WLOs can also be utilized to study human lung development, including signaling between the lung epithelium and mesenchyme, to model genetic mutations on human lung cell function and development, and to determine the cytotoxicity of infective agents.
Introduction
The lung is a complicated, heterogeneous, dynamic organ that develops in six distinct stages – embryonic, pseudoglandular, canalicular, saccular, alveolar, and microvascular maturation1,2. The latter two phases occur pre and postnatally in human development while the first four stages occur exclusively during fetal development unless preterm birth occurs3. The embryonic phase begins in the endodermal germ layer and concludes with the budding of the trachea and lung buds. Lung development occurs in part via signaling between the epithelial and mesenchymal cells4. These interactions result in lung branching, proliferation, cellular fate determination and cellular differentiation of the developing lung. The lung is divided into conducting zones (trachea to the terminal bronchioles) and respiratory zones (respiratory bronchioles to the alveoli). Each zone contains unique epithelial cell types; including basal, secretory, ciliated, brush, neuroendocrine, and ionocyte cells in the conducting airway5, followed by alveolar type I and II cells in the respiratory epithelium6. Much is still unknown about the development and response to injury of the various cell types. iPSC derived lung organoid models enable the study of mechanisms that drive human lung development, the effects of genetic mutations on pulmonary function, and the response of both the epithelium and mesenchyme to infectious agents without the need for primary human lung tissue.
Markers corresponding to the various stages of embryonic differentiation include CXCR4, cKit, FOXA2, and SOX17 for definitive endoderm (DE)7, FOXA2, TBX1, and SOX2 for anterior foregut endoderm (AFE)8, and NKX2-1 for early lung progenitor cells9. In embryonic lung development, the foregut divides into the dorsal esophagus and ventral trachea. The buds of the right and left lungs appear as two independent outpouchings around the tracheal bud10. During branching morphogenesis, the mesenchyme surrounding the epithelium produces elastic tissue, smooth muscle, cartilage, and vasculature11. The interaction between the epithelium and mesenchyme is essential for normal lung development. This includes the secretion of FGF1012 by the mesenchyme and SHH13 produced by the epithelium.
Here, we describe a protocol for the directed differentiation of hiPSCs into three-dimensional (3D) whole lung organoids (WLO). While there are similar approaches that incorporate isolation of lung progenitor cells via sorting at the LPC stage to make alveolar-like14,15 (distal) organoids or airway16 (proximal) organoids, or generate ventral-anterior foregut spheroids to make human lung organoids expressing alveolar-cell and mesenchymal markers and bud tip progenitor organoids17, the strength of this method is the inclusion of both lung epithelial and mesenchymal cell types to pattern and orchestrate lung branching morphogenesis, maturation, and expansion in vitro.
This protocol uses small molecules and growth factors to direct the differentiation of pluripotent stem cells through definitive endoderm, anterior foregut endoderm, and lung progenitor cells. These cells are then induced into 3D whole lung organoids through important developmental steps, including branching and maturation. The summary of the differentiation protocol is shown in Figure 1a with representative brightfield images of endodermal and organoid differentiation shown in Figure 1b. Figure 1c,d show the gene expression details of endodermal differentiation as well as the gene expression of both the proximal and distal populations of lung epithelial cells after completing the differentiation.
Protocol
This study protocol was approved by the Institutional Review Board of UCSD's Human Research Protections Program (181180).
1. Definitive endoderm induction from induced pluripotent stem cells (Day 1 – 3)
- Slowly thaw growth factor reduced (GFR)-basement membrane (BM) matrix medium on ice 30 minutes prior to use. In cold DMEM/F12, mixture, dilute the GFR BM matrix medium 1:1 such that it constitutes 50% of this medium. Place P1000 pipette tips in the freezer to chill prior to use.
- Coat each well of a 12-well plate with 500 µL of 50% GFR-basement membrane matrix medium prepared in ice-cold DMEM/F12. Once the desired number of wells are coated, remove any excess medium mixture and/or bubbles from wells and place the plate on ice or refrigerator at 4 °C for 20 min to set. Then, move the plate to the incubator at 37 °C overnight to gel and dry.
- Once hiPSCs reach 70-90% confluency, add 10 µM of Rho-associated kinase (ROCK) Inhibitor Y-27632 an hour prior to dissociation. Aspirate off the media and wash once with Phosphate buffered saline (PBS). Dissociate hiPSCs by adding cell detachment medium (0.5 mL/ well of a 12-well plate) and incubate for 20 min at 37 °C in a 5% CO2 incubator.
- Remove plates from the incubator and add 0.5 mL/12-well of stem cell passaging medium (Table 1) to the wells; gently triturate cells using a P1000 tip to obtain single-cell suspension. Transfer dissociated cells into a 15 mL conical centrifuge tube; centrifuge for 5 min at 300 x g.
- Aspirate off the medium and resuspend the cell pellet with 1 mL of mTeSR Plus media supplemented with 10 µM ROCK inhibitor (Y-27632). Perform a cell count. Add 2.0 x 105 hiPSCs in 1 mL of mTeSR supplemented with ROCK Inhibitor Y-27632 per well of a 12-well GFR-basement membrane medium coated plate. Incubate at 37 °C overnight.
NOTE: Cell seeding number must be optimized per cell line. 24 h after plating, wells should be 50%-70% confluent. - On day 1, aspirate off the mTeSR Plus and add Definitive Endoderm (DE) induction media (Table 1) supplemented with 100 ng/mL of human activin A and 5 µM of GSK3β inhibitor/Wnt activator CHIR99021.
NOTE: DE media with GSK3β inhibitor/Wnt activator CHIR99021 should be removed within 20-24 h of day 1 DE induction for successful differentiation. - On day 2 and day 3, change to DE induction media supplemented with 100 ng/mL of activin A only.
NOTE: DE differentiation should not exceed a total of 72 h, or efficacy will decrease. On day 4, if large cell die-off is observed, decrease total DE media exposure time by 6-12 h. - To analyze DE efficiency, confirm greater than 90% CXCR4 and/or cKit expression via flow cytometry or immunofluorescence analysis of FOXA2 and/or SOX17 (Figure 2a).
2. Anterior foregut endoderm (AFE) induction (Day 4 – 6)
- On day 4, change media to serum free basal medium (SFBM) (Table 1) supplemented with 10 µM SB431542 and 2 µM Dorsomorphin for AFE induction. Change AFE media daily for 3 days (day 4, day 5, and day 6).
- To analyze AFE efficiency, confirm robust expression of SOX2, TBX1, and FOXA2 via immunofluorescence staining (Figure 2b).
3. Lung progenitor cell (LPC) differentiation (Day 7 – 16)
- On day 7, thaw GFR-basement membrane matrix medium on ice. Aspirate off the AFE media and wash well with 1x PBS. Add 1 mL of cell detachment solution and incubate for 10 min at 37 °C.
- Add 1 mL of quenching medium (2% FBS in DMEM/F12) to the wells containing cell detachment solution. Keep cells as aggregates by pipetting up and down gently. Make sure all cells are dislodged and transferred into a 15 mL conical centrifuge tube. Centrifuge for 5 min at 300 x g.
- Remove the supernatant and resuspend the cell pellet in Quenching medium supplemented with 10 ng/mL of human recombinant bone morphogenic protein-4 (BMP4), 0.1 µM of all-trans retinoic acid (RA), 3 µM of GSK3β inhibitor/Wnt activator CHIR99021, and 10 µM of Rock Inhibitor Y-27632.
- Perform a cell count. Add 2.5 x 105 cells to 100 µL of cold GFR-basement membrane matrix medium, mix well, and place droplet into a well of a 12-well plate. Incubate the plate at 37 °C for 30-60 min to allow the medium to polymerize. Add 1 mL of LPC media supplemented with 10 µM of ROCK Inhibitor Y-27632 per well ensuring the medium drop is fully submerged and incubate at 37 °C overnight.
- On day 8, 24 h after LPC induction, change LPC medium to remove ROCK Inhibitor Y-27632. Change the LPC medium every other day for a total of 9-11 days.
NOTE: If the medium becomes yellow within 24 h, change medium every day. - To analyze LPC efficiency, confirm robust expression of the intracellular transcription factor NKX2-1 or perform flow cytometry for surface markers CD47hi/CD26low15 or CPM18 (Figure 2c). Grossly, the LPC spheroids should be round and transparent (Figure 2c).
NOTE: Do not proceed with lung organoid differentiation if efficiency of NKX2-1 is below 30%.
4. 3D lung organoid induction (Day 16 – 22)
- On day 17, thaw GFR-basement membrane matrix medium on ice. Aspirate the LPC induction medium. Then add 2 µg/mL of dispase (1 mL) to the well and resuspend the medium/dispase mixture with a P1000 pipette. Incubate at 37 °C for 15 min. Triturate the mixture again and incubate at 37 °C for another 15 min.
- Transfer the dispase and cells into a 15 mL conical centrifuge tube. Use chilled PBS (2-3 mL) to wash the well and resuspend the dispase/cell mixture. Centrifuge for 5 min at 400 x g. Manually remove the supernatant, being careful not to distub the medium/cell pellet layer. Repeat the chilled PBS wash, and centrifuge the conical centrifuge tube for another 5 min at 400 x g.
- Manually remove the supernatant, and then add 2 mL of trypsin- based dissociation solution to the conical centrifuge tube. Incubate at 37 °C for 12 min.
- After incubation, resuspend cells with a P1000 pipette tip. Then add an equal volume of quenching media to the conical centrifuge tube and spin down at 400 x g for 5 min. Aspirate the supernatant and resuspend cells in quenching medium + 10 µM of ROCK Inhibitor Y-27632.
NOTE: Successful lung organoid induction occurs when cells are embedded as aggregates, not single cells, adjust pipetting accordingly. - Perform a cell count. Calculate the volume needed to obtain 5.0-8.0 x 104 cells per well. Aliquot the LPC cell aggregates into 1.5 mL microcentrifuge tubes and centrifuge for 5 min at 400 x g. Remove excess supernatant, being careful to not agitate the cell pellet. Leave only 10 µL of residual media.
- Re-suspend the cell pellet in 200 µL of cold GFR-basement membrane matrix medium and add to cell culture membrane inserts (6.5 mm diameter, 0.4 µm pore, polyester membrane). Incubate the plate at 37 °C for 30-60 min to allow GFR-basement membrane matrix medium to polymerize.
- Add 1 mL of 3D organoid induction medium (Table 1) supplemented with fibroblast growth factor-7 (FGF7) (10 ng/mL), FGF10 (10 ng/mL), GSK3β inhibitor/Wnt activator CHIR (3 µM), epidermal growth factor (EGF) (10 ng/mL), and 10 µM of ROCK Inhibitor Y-27632 to the basolateral chamber of the membrane insert. Change the medium every other day for 6 days.
5. 3D Lung organoid branching (Day 23 – 28)
- On day 23 change to 3D branching medium (Table 1) supplemented with FGF7 (10 ng/mL), FGF10 (10 ng/mL), GSK3β inhibitor/Wnt activator CHIR99021 (3 µM), RA (0.1 µM), EGF (10 ng/mL), and vascular endothelial growth factor (VEGF) / placental growth factor (PlGF) (10 ng/mL). Change the medium every other day for 6 days.
NOTE: At day 6 of 3D branching differentiation, there should be multiple branching organoids (Figure 2).
6. 3D lung organoid maturation (Day 29 – 34)
- On day 29, change to 3D maturation medium (Table 1), which is the same as 3D branching medium but with the addition of dexamethasone (50 nM), cAMP (100 µM), and and 3-isobutyl-1-methylxanthine (IBMX), a phosphodiesterase inhibitor also termed isobutylxanthine (100 µM). Change the medium every other day for 6 days.
NOTE: Within 24 h after 3D maturation, the branching organoids should expand and change into transparent spheres.
7. 3D Lung organoid immunocytochemistry
- For 3D whole lung organoid analysis, fix GFR-basement membrane matrix medium in the membrane inserts with 4% paraformaldehyde (PFA) for 1 h at 4 °C. Embed in paraffin wax and mount onto slides per standard published protocols.
- Perform antigen retrieval prior to staining. Airway markers include KRT5, MUC5AC, and SCGB3A2. Alveolar markers include SP-C, SP-B, HTII-280, HTI-56, and HOPX (Figure 3).
8. Removal of whole lung organoids from GFR-basement membrane matrix medium for passage, FACS, or cryopreservation
- To dissociate the organoids from the GFR-basement membrane matrix medium, remove media from the basal chamber and add 2 µg/mL of dispase (1 mL) in the apical chamber.
- Gently triturate the medium /dispase mixture with a P1000 pipette and place in the incubator for 15 min. Gently triturate the mixture again and incubate for another 15 min.
- Add 1 mL of chilled PBS (4 °C) and transfer organoids with the matrix medium into a 15 mL conical centrifuge tube. Spin at 400 x g for 5 min. Remove the supernatant carefully, not to disturb the cell pellet.
- Wash once more with 1 mL chilled PBS and spin down at 400 x g for 5 min. Remove the supernatant carefully, not to disturb the medium/cell mixture.
- Add 1 mL of cell detachment solution to the conical centrifuge tube, gently triturate resuspend the GFR-Basement membrane matrix medium/cell mixture. Place in the incubator for 12 min for passaging cells as aggregates or for cryopreservation), or 20 min for single cell suspension.
- Add equal volume of quenching media and spin down at 400 x g for 5 min. Resuspend in quenching medium + 10 µM of ROCK Inhibitor Y-27632.
NOTE: At this step, no residual basement membrane medium should be seen in the tube. If residual medium remains, repeat steps 8.5 and 8.6. - Perform a cell count. Calculate the volume needed for downstream applications.
Representative Results
24 hours after plating, day 1, iPSCs should be 50%-90% confluent. On day 2, DE should be 90%-95% confluent. During DE induction, it is common to observe significant cell death on day 4 but attached cells will retain a compact cobblestone morphology (Figure 2b). Discontinue differentiation if the majority of adherent cells detach and consider shortening exposure to DE media with activin A by 6-12 h. During AFE induction, cell death is minimal, and cells remain adherent, but will appear smaller and more heterogeneous. Passaging the cells on day 7 must only be done if the yield of double positive SOX2 and FOXA2 is >80%. After passaging into basement membrane matrix for 3D LPC induction, small spheroids will first appear, then grow and some may begin to branch. Gene expression profiles for successful endodermal differentiation include increased SOX17 at DE, increased FOXA2 and SOX2 with decreasing SOX17 and the first appearance of NKX2-1, and increased NKX2-1, along with the presence of SOX2 and FOXA2. Consistent with early embryonic development, the ventralization of AFE occurs for lung bud development (NKX2-1+) and dorsalization of AFE occurs for gastrointestinal development (SOX2+). Cultures at LPC will have a mix of both lung and gastric progenitors.
Lung organoid induction from LPC has been performed using various methods. Some groups sort the cells using NKX2-1 fluorescent reporters or a surface antigen proxy (CPM, CD26lowCD47high). But those lung organoids contain alveolar type II like cells without alveolar type I cells or mesenchyme. Other groups have collected cell clumps that bud off the AFE/LPC monolayer and embedded them into basement membrane matrix. Those organoids contain a mixed population of lung epithelial and mesenchymal cells but take months to culture19. Our protocol includes both the presence of epithelial and mesenchymal cells. The WLOs express proximal epithelial cell markers p63 and KRT5 (basal cells) and SCGB3A2 (club cells) as well as distal epithelial cell markers HOPX (ATI) and proSPC, SPB, and NKX2-1 (ATII). They also express the mesenchymal marker Vimentin at the LPC stage, as well as in the whole lung organoids. PDGFRα is a marker for fibroblasts that have an important function in the lung during sacculation and alveolarization20 and is co-expressed with the transcription factor important in distal cell differentiation, SOX9 (Figure 3).
Our method efficiently generates NKX2-1-expressing LPC 3D cultures using signaling molecules that occur in fetal lung development to form early lung organoids. When passaging LPCs into GFR-basement membrane matrix medium for lung organoid induction, it is imperative not to over-dissociate into a single cell suspension, but to instead to retain small clumps of cells (10 cells/clump). Cell counting will not be completely accurate, but still necessary to avoid over confluence during the 3-week lung organoid differentiation.
Lung organoid induction should yield small, branching organoids by day 6 of induction (day 23 of differentiation). These should continue to grow during the organoid branching step and maturation step. Twenty-four hours after the introduction of dexamethasone, cAMP, and IBMX, the branches should expand into transparent spheres. Whole lung organoid analysis can be performed at the end of the differentiation, or the WLOs can be passage into fresh basement membrane matrix with GFR or cryopreserved by freezing down in 10% DMSO.
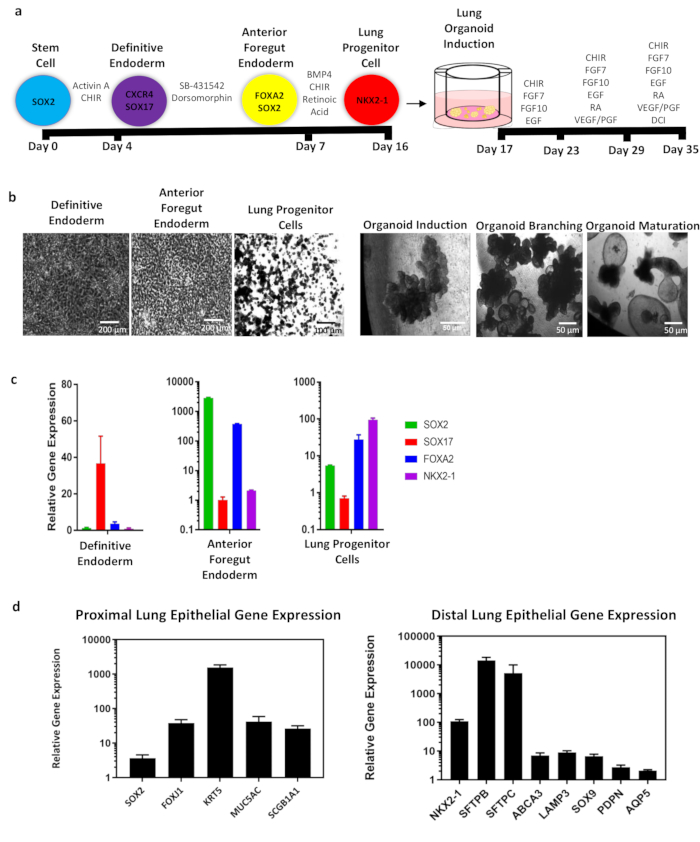
Figure 1: Overall schematic of whole lung organoid (WLO) differentiation from hiPSCs and representative data. (a) Schematic of WLO differentiation from hiPSCs. Circles represent endodermal cell type with identifying markers. Timeline of differentiation is indicated in black bars. Growth factors and/or small molecules for induction of endodermal and lung organoid populations. In summary, stem cells are differentiated into definitive endoderm, anterior foregut endoderm and into lung progenitor cells in approximately 16 days. These cells are then passage into GFR-basement membrane matrix medium containing medium inserts and undergo lung organoid induction, branching, and maturation. The total differentiation takes approximately 35 days. (b) Representative phase contrast images of the cells at major endodermal stages and 3D images of whole lung organoids. Scale bar size as indicated in panel. (c) qRT-PCR analysis of lung development markers during endoderm and (d) whole lung organoid differentiation of proximal and distal cell markers. All data represents an average of 3-5 biological replicates. Error bars represent standard error of the mean and are normalized to actin. Please click here to view a larger version of this figure.
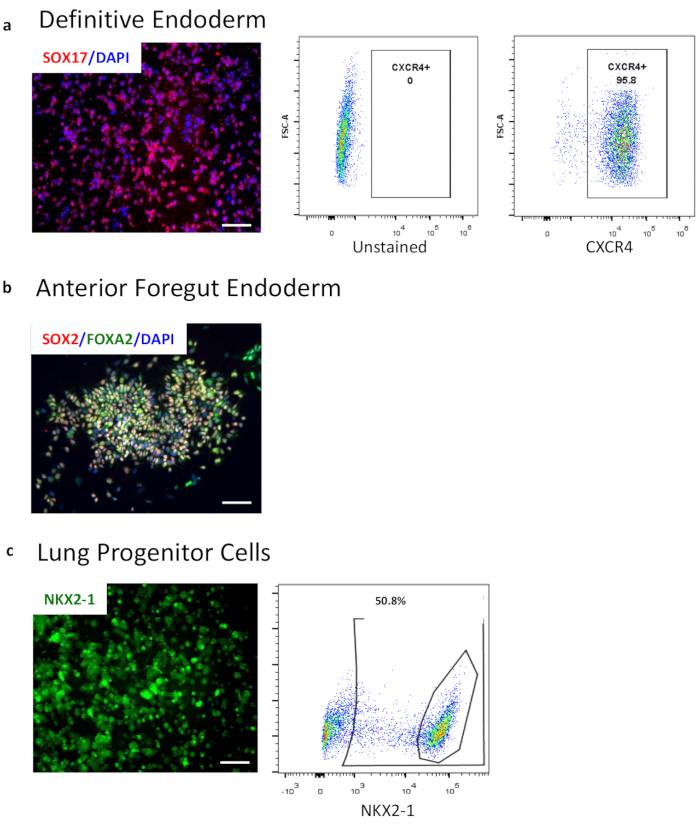
Figure 2: Characterization of endoderm differentiation by flow cytometry and immunocytochemistry. (a) Flow cytometry of definitive endoderm marker CXCR4. Left panel shows the gating against the unstained population while the middle panel shows the CXCR4 positive population. The right panel shows immunocytochemistry image of SOX17 (red) overlaid with nuclei (blue). (b) Immunocytochemistry image of AFE markers FOXA2 and SOX2 overlaid with nuclei (blue). (c) Endogenous expression of NKX2-1-GFP in a reporter cell line in 3D LPC. Images taken from live cell culture in brightfield and GFP. Flow cytometry of lung progenitor intracellular marker NKX2-1 after fixation and permeabilization. Scale bar size = 50 µM. Please click here to view a larger version of this figure.
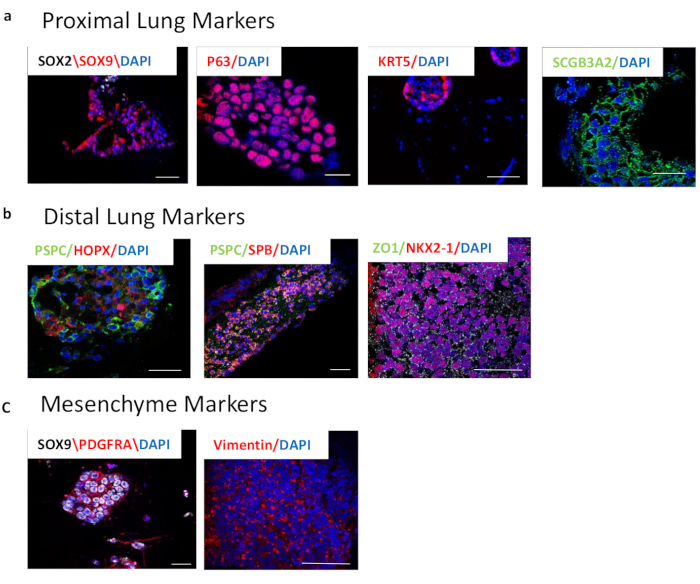
Figure 3: Characterization of 3D whole lung organoids after 3-week differentiation by immunocytochemistry. (a) Proximal lung markers. Left panel shows SOX2 (white) and SOX9 (red) overlaid by nuclei (blue). These markers are important in branching morphogenesis and represent the proximal and distal epithelial populations. Middle panels show P63 (red) and KRT5 (red), both markers of basal cells. The right panel shows SCGB3A2, a marker of club cells. (b) Distal lung markers. Left panel depicts pro-SPC (PSPC), (green) and HOPX (red), markers of alveolar type II ad I cells, respectively, overlaid with nuclei (blue). Middle panel shows pro-SPC (PSPC) (green) and SPB (red), markers of alveolar type II cells, overlaid with nuclei (blue). The right panel shows NKX2-1 (red) and ZO1 (green) overlaid with nuclei (blue). (c) Markers of lung mesenchyme. Left panel shows PDGFRA (red) and SOX9 (white), representing distal mesenchyme. Right panel shows Vimentin (red), which is dispersed throughout the lung. Scale bar size = 50 µM. Please click here to view a larger version of this figure.
| REAGENTS AND SOLUTIONS – For company names please see the Table of Materials List |
| 3D organoid induction medium (day 17-22) |
| Serum-free basal medium (see recipe) supplemented with: |
| FGF7 (10 ng/mL) |
| FGF10 (10 ng/mL) |
| CHIR99021 (3 μM) |
| EGF (10 ng/mL) |
| 3D organoid branching medium (day 23-28) |
| Serum-free basal medium (see recipe) supplemented with: |
| FGF7 (10 ng/mL) |
| FGF10 (10 ng/mL) |
| CHIR99021 (3 μM) |
| All-trans retinoic acid (0.1 μM) |
| EGF (10 ng/mL) |
| VEGF/PIGF (10 ng/mL) |
| 3D organoid maturation medium (day 29-34) |
| Serum-free basal medium (see recipe) supplemented with: |
| Dexamethasone (50 nM) |
| Br-cAMP (100 μM) |
| IBMX (100 μM) |
| AFE induction medium (day 4-6) |
| Serum-free basal medium (see recipe) supplemented with: |
| SB431542 (10 μM) |
| Dorsomorphin (2 μM) |
| DE induction medium (day 1-3) |
| 48.5 mL RPMI1640 + Glutamax |
| 1 mL B27 without retinoic acid |
| 500 μl HEPES (1%) |
| 500 μl pen/strep |
| Human activin A (100 ng/mL) |
| CHIR99021 (5 μM) – only in the first 24 hours |
| LPC induction medium (day 7-16) |
| Serum-free basal medium (see recipe) supplemented with: |
| BMP4 (10 ng/mL) |
| All-trans retinoic acid (RA) (0.1 μM) |
| CHIR99021 (3 μM) |
| Quenching medium |
| 49 mL DMEM/F12 |
| 1 mL FBS |
| Serum-free basal medium (SFBM) |
| 375 mL Iscove’s Modified Dulbecco’s Medium (IMDM) + Glutamax |
| 125 mL Ham’s F12 |
| 5 mL B27 without retinoic acid |
| 2.5 mL N2 |
| 500 μl ascorbic acid, 50 mg/mL |
| 13 μl monothioglycerol/1ml of IMDM” use 300ul of 0.4mM monothioglycerol per 100ml of serum free media |
| 3.75 mL bovine serum albumin (BSA) Fraction V, 7.5% solution |
| 500 μl pen/strep |
| Stem cell passaging medium (day 0) |
| 500 mL DMEM/F12 |
| 129 mL Knockout serum replacement (KSR) |
| 6.5 mL Glutamax |
| 6.5 mL NEAA |
| 1.3 mL 2-mercaptoethanol |
| 6.5 mL pen/strep |
Table 1: Table of media.
| Problem | Solution |
| DE differentiation not efficient | 24 hours after plating in stem cell medium, cells should be 50-70% confluent |
| GSK3β inhibitor/Wnt activator CHIR99021 should be removed within 20-24 hours of day 1 DE induction | |
| DE differentiation should not exceed a total of 72 hours | |
| AFE differentiation not efficient | Ensure that DE differentiation was successful and the cells express > 80% CXCR4 |
| Ensure fresh growth factors/small molecules are being added to the media daily | |
| LPC differentiation not efficient | Ensure the AFE differentiation was successful and the cells express > 80% FOXA2/SOX2 |
| Ensure the AFE cells are passaged as aggregates of 4-10 cells and not single celled | |
| 3D lung organoids not growing or differentiating | Ensure the LPC differentation was successful and the cells express > 30% NKX2-1 |
| Ensure the LPCs were passaged as aggregates of 4-10 cells and not single celled | |
| Ensure there is no residual matrigel from the LPCs during passaging | |
| Add ROCK Inhibitor Y-27632 with each media change | |
| Ensure the media is changed on time and fresh growth factors/small molecules are added | |
| Ensure concentration of growth factors/small molecules is correct |
Table 2: Troubleshooting.
Discussion
The successful differentiation of 3D whole lung organoids (WLO) relies on a multi-step, 6-week protocol with attention to detail, including time of exposure to growth factors and small molecules, cellular density after passaging, and the quality of hiPSCs. For troubleshooting, see Table 2. hiPSCs should be approximately 70%-80% confluent, with less than 5% spontaneous differentiation prior to dissociation. This protocol calls for "mTeSR plus" medium; however, plain "mTeSR" medium has also been used with comparable results and is less expensive. For the extracellular matrix, we use GFR basement membrane matrix medium. We passage the hiPSCs using ReLesR (see Table of Materials) to reduce differentiation.
During endoderm differentiation, cells should be visualized daily prior to and after media changes. Specified growth factors/small molecules should be added fresh daily to the base medium to prevent premature degradation. Cell death in definitive endoderm (DE) is common but should be limited during anterior foregut endoderm (AFE) and lung progenitor cell (LPC) induction. If there is a large die off on the third day of DE (day 4), decrease total time of DE by 6-12 h. New iPSC cell lines may need to be optimized for successful endoderm differentiation. Perform flow cytometry at DE for CXCR4 to confirm successful induction (>85% CXCR4 + cells). The cells should be relatively stable at AFE and will change morphologically with little die off.
Passaging into LPC is another process that must be optimized for cell type. Replating cells at too low a density (<50%) will result in inefficient differentiation. Confirm successful LPC induction with immunocytochemistry for NKX2-1 or flow cytometry for CPM18 or CD26low/CD47high 15. Successful LPC induction must have >40% NKX2-1, otherwise the organoids will have greater abundance of dorsal AFE. For LPC induction, growth factors must be added to base media with each media change. If the media becomes yellow later into LPC induction, consider increasing the volume of fresh media, or change the media every day. During 3D whole lung organoid induction, plating number and maintaining cell clusters are key to successful organoid growth. GFR-basement membrane matrix medium is difficult to handle and highly temperature sensitive, so always keep it on ice. If the GFR-basement membrane matrix medium gels too early, then the LPC cells will not integrate within it. We recommend thawing 1 mL aliquots of GFR-basement membrane matrix medium on ice 30 min prior to passaging. Once the cells/clusters have been counted and appropriate aliquots made, place the cell pellet on ice. We suggest preparing plates, labeling, and addition of cell culture membrane inserts prior to GFR-basement membrane matrix medium handling.
Use pipette tips to add correct volume of liquid GFR-basement membrane matrix medium immediately to cell pellet, keeping on ice. Pipette up and down quickly but gently (nuanced handling) to not introduce bubbles, then place the tube back on ice. Add the cell and GFR-basement membrane matrix medium mixture to the apical portion of the transwell in prepared plates. The mixture should spread and coat the entire transwell; gently tilt the plate to ensure coating. After gelling in the incubator for 30-60 min, there should be visible cell clusters in the GFR-basement membrane matrix medium. Add appropriate lung induction media to the basal chamber supplemented with 10 µM ROCK Inhibitor Y-27632 and monitor organoid growth every other day.
Future applications of the organoids generated by this protocol include studying the molecular pathways that control early lung lineage commitment and cell fate specification21,22,23. The interaction between the epithelium and mesenchyme can be determined by utilizing gene knock out models24. The organoids could also be co-cultured with endothelial cells to determine the importance of tissue specific co-pattern signaling between the lung epithelium, mesenchyme, and the endothelium25. Lung development occurs in parallel with vascular development and that relationship may elicit important molecular mechanisms necessary for proper lung development. We have also shown that these whole lung organoids are functional through surfactant secretion assays after GFR-basement membrane matrix medium was removed followed by short-term culture in ultra-low attachment wells26. Other strategies include sorting the cells for cell surface markers such as NGFR (basal cells)27 and HTII-280 (ATII cells)28 and replacing them as homogenous organoids or a monolayer in air liquid interphase culture conditions. Whole, proximal, and distal lung organoids have also been used to study the cellular targets and pathophysiology of SARS-CoV-2 viral infection in order to better understand and screen for drugs that might combat COVID-19.
This protocol is robust and reproducible, but many challenges still exist. Many different iPSC and ESC lines have been tested (>20 lines) but the protocol must be optimized for each cell line. Despite robust DE and AFE induction, LPC induction may be difficult to achieve >40% of NKX2-1 + cells. Other protocols include a sorting step for surface markers of NKX2-1 cells15,18, but those only yield alveolar type II like organoids without mesenchyme and still contain gastric and hepatic cell populations despite purifying the lung progenitor population29. We have also noted a small amount of gastric and hepatic cells in both the LPC and whole lung organoids, possibly due to the presence of dorsal anterior foregut cells contaminating the LPCs. Therefore, the differentiation of pure lung organoids is yet to be achieved, and more research on the development of the lung progenitor cells in human tissue must be completed. Downstream assays must be vigorously benchmarked with gene and protein expression from primary human lung tissue. While, to date, the most fruitful use of lung organoids has been in modeling diseases and screening for drugs in vitro, the transplantation of hiPSC-derived lung organoids into patients for regenerative medicine has been contemplated as a future therapy for a range of conditions. However, prior to considering such interventions, a good deal of quality control must be perfected, including the identification and removal of contaminating, undesirable, potentially tumorigenic hiPSC derivatives. Furthermore, better functional assays in vitro and better animal models of pulmonary disease still need to be developed.
Specifically, the functionality and safety of hiPSC-derived cells must be confirmed. Undifferentiated cells need to be excluded since they have the capacity to generate teratomas. One method to determine undifferentiated stem cells in definitive endoderm is to sort the cells out using the pluripotency marker SSEA4. Marker genes for undifferentiated hiPSCs were recently detected using single cell RNA sequencing30. ESRG, CNMD, and SFRP2 can be used to validate undifferentiated cells at any differentiation step. Once purity is confirmed, the benefit of autologous iPSC derived therapies is the ability for the transplanted cells to avoid rejection since they come from the patient's own cells. The drawbacks include the time it takes to fully differentiate the cells, undergo rigorous clinical grade testing, and transplant the cells into a patient with an acute injury (respiratory distress syndrome, myocardial infarction, or spinal injury). The alternative is to utilize banked allogenic iPSC derived cells31. These may be stored and readily available for patients with human leukocyte antigen (HLA) matched donors and they will have undergone thorough testing for contamination. The biggest drawback is the possibility of immune rejection. Immunosuppression may be necessary in allogenic cell transplantation, which is the current reality of allogenic whole tissue transplants. Strategies are being devised to allow the allogenic iPSC derived cells to evade the immune response to safely transplant them into patients32.
Eventually, iPSC derived whole lung organoids will be utilized to study patient-specific disease models, tailor therapeutics, and enhance regenerative medical research.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was supported by the California Institute for Regenerative Medicine (CIRM) (DISC2-COVID19-12022).
Materials
| Cell Culture | |||
| 12 well plates | Corning | 3512 | |
| 12-well inserts, 0.4um, translucent | VWR | 10769-208 | |
| 2-mercaptoethanol | Sigma-Aldrich | M3148 | |
| Accutase | Innovative Cell Tech | AT104 | |
| ascorbic acid | Sigma | A4544 | |
| B27 without retinoic acid | ThermoFisher | 12587010 | |
| Bovine serum albumin (BSA) Fraction V, 7.5% solution | Gibco | 15260-037 | |
| Dispase | StemCellTech | 7913 | |
| DMEM/F12 | Gibco | 10565042 | |
| FBS | Gibco | 10082139 | |
| Glutamax | Life Technologies | 35050061 | |
| Ham’s F12 | Invitrogen | 11765-054 | |
| HEPES | Gibco | 15630-080 | |
| Iscove’s Modified Dulbecco’s Medium (IMDM) + Glutamax | Invitrogen | 31980030 | |
| Knockout Serum Replacement (KSR) | Life Technologies | 10828028 | |
| Matrigel | Corning | 354230 | |
| Monothioglycerol | Sigma | M6145 | |
| mTeSR plus Kit (10/case) | Stem Cell Tech | 5825 | |
| N2 | ThermoFisher | 17502048 | |
| NEAA | Life Technologies | 11140050 | |
| Pen/strep | Lonza | 17-602F | |
| ReleSR | Stem Cell Tech | 5872 | |
| RPMI1640 + Glutamax | Life Technologies | 12633012 | |
| TrypLE | Gibco | 12605-028 | |
| Y-27632 (Rock Inhibitor) | R&D Systems | 1254/1 | |
| Growth Factors/Small Molecules | |||
| Activin A | R&D Systems | 338-AC | |
| All-trans retinoic acid (RA) | Sigma-Aldrich | R2625 | |
| BMP4 | R&D Systems | 314-BP/CF | |
| Br-cAMP | Sigma-Aldrich | B5386 | |
| CHIR99021 | Abcam | ab120890 | |
| Dexamethasone | Sigma-Aldrich | D4902 | |
| Dorsomorphin | R&D Systems | 3093 | |
| EGF | R&D Systems | 236-EG | |
| FGF10 | R&D Systems | 345-FG/CF | |
| FGF7 | R&D Systems | 251-KG/CF | |
| IBMX (3-Isobtyl-1-methylxanthine) | Sigma-Aldrich | I5879 | |
| SB431542 | R&D Systems | 1614 | |
| VEGF/PIGF | R&D Systems | 297-VP/CF | |
| Primary antibodies | Dilution rate | ||
| CXCR4-PE | R&D Systems | FAB170P | 1:200 (F) |
| HOPX | Santa Cruz Biotech | sc-398703 | 0.180555556 |
| HTII-280 | Terrace Biotech | TB-27AHT2-280 | 0.145833333 |
| KRT5 | Abcam | ab52635 | 0.180555556 |
| NKX2-1 | Abcam | ab76013 | 0.25 |
| NKX2-1-APC | LS-BIO | LS-C264437 | 1:1000 (F) |
| proSPC | Abcam | ab40871 | 0.215277778 |
| SCGB3A2 | Abcam | ab181853 | 0.25 |
| SOX2 | Invitrogen | MA1-014 | 0.180555556 |
| SOX9 | R&D Systems | AF3075 | 0.180555556 |
| SPB (mature) | 7 Hills | 48604 | 1: 1500 (F) 1:500 (W)a |
| SPC (mature) | LS Bio | LS-B9161 | 1:100 (F); 1:500 (W) a |
References
- Ten Have-Opbroek, A. A. Lung development in the mouse embryo. Experimental Lung Research. 17 (2), 111-130 (1991).
- Perl, A. K., Whitsett, J. A. Molecular mechanisms controlling lung morphogenesis. Clinical Genetics. 56 (1), 14-27 (1999).
- Leibel, S., Post, M. Endogenous and exogenous stem/progenitor cells in the lung and their role in the pathogenesis and treatment of pediatric lung disease. Frontiers in Pediatrics. 4, 36 (2016).
- Hines, E. A., Sun, X. Tissue crosstalk in lung development. Journal of Cellular Biochemistry. 115 (9), 1469-1477 (2014).
- Montoro, D. T., et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 560 (7718), 319-324 (2018).
- Barkauskas, C. E., et al. Type 2 alveolar cells are stem cells in adult lung. The Journal of Clinical Investigation. 123 (7), 3025-3036 (2013).
- D’Amour, K. A., et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnology. 23 (12), 1534-1541 (2005).
- Green, M. D., et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nature Biotechnology. 29 (3), 267-272 (2011).
- Ikeda, K., Shaw-White, J. R., Wert, S. E., Whitsett, J. A. Hepatocyte nuclear factor 3 activates transcription of thyroid transcription factor 1 in respiratory epithelial cells. Molecular Cell Biology. 16 (7), 3626-3636 (1996).
- Schittny, J. C. Development of the lung. Cell and Tissue Research. 367 (3), 427-444 (2017).
- Mecham, R. P. Elastin in lung development and disease pathogenesis. Matrix Biology: Journal of the International Society for Matrix Biology. 73, 6-20 (2018).
- Bellusci, S., Grindley, J., Emoto, H., Itoh, N., Hogan, B. L. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 124 (23), 4867-4878 (1997).
- Bellusci, S., et al. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 124 (1), 53-63 (1997).
- Yamamoto, Y., et al. Long-term expansion of alveolar stem cells derived from human iPS cells in organoids. Nature Methods. 14 (11), 1097-1106 (2017).
- Hawkins, F., et al. Prospective isolation of NKX2-1-expressing human lung progenitors derived from pluripotent stem cells. The Journal of Clinical Investigation. 127 (6), 2277-2294 (2017).
- McCauley, K. B., Hawkins, F., Kotton, D. N. Derivation of epithelial-only airway organoids from human pluripotent stem cells. Current Protocols in Stem Cell Biology. 45 (1), 51 (2018).
- Miller, A. J., et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nature Protocols. 14 (2), 518-540 (2019).
- Gotoh, S., et al. Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports. 3 (3), 394-403 (2014).
- Huang, S. X., et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nature Biotechnology. 32 (1), 84-91 (2014).
- Endale, M., et al. Temporal, spatial, and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Biologie du développement. 425 (2), 161-175 (2017).
- Fatehullah, A., Tan, S. H., Barker, N. Organoids as an in vitro model of human development and disease. Nature Cell Biology. 18 (3), 246-254 (2016).
- Nikolić, M. Z., Rawlins, E. L. Lung organoids and their use to study cell-cell interaction. Current Pathobiology Reports. 5 (2), 223-231 (2017).
- Calvert, B. A., Ryan Firth, A. L. Application of iPSC to modelling of respiratory diseases. Advances on Experimental Medicine and Biology. 1237, 1-16 (2020).
- McCulley, D., Wienhold, M., Sun, X. The pulmonary mesenchyme directs lung development. Current Opinion in Genetics and Development. 32, 98-105 (2015).
- Blume, C., et al. Cellular crosstalk between airway epithelial and endothelial cells regulates barrier functions during exposure to double-stranded RNA. Immunity, Inflammation and Disease. 5 (1), 45-56 (2017).
- Leibel, S. L., et al. Reversal of surfactant protein B deficiency in patient specific human induced pluripotent stem cell derived lung organoids by gene therapy. Scientific Reports. 9 (1), 13450 (2019).
- Rock, J. R., et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences of the United States of America. 106 (31), 12771-12775 (2009).
- Gonzalez, R. F., Allen, L., Gonzales, L., Ballard, P. L., Dobbs, L. G. HTII-280, a biomarker specific to the apical plasma membrane of human lung alveolar type II cells. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 58 (10), 891-901 (2010).
- McCauley, K. B., et al. Single-cell transcriptomic profiling of pluripotent stem cell-derived SCGB3A2+ airway epithelium. Stem Cell Reports. 10 (5), 1579-1595 (2018).
- Sekine, K., et al. Robust detection of undifferentiated iPSC among differentiated cells. Scientific Reports. 10 (1), 10293 (2020).
- Jacquet, L., et al. Strategy for the creation of clinical grade hESC line banks that HLA-match a target population. EMBO Molecular Medicine. 5 (1), 10-17 (2013).
- Mattapally, S., et al. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell-derived cells: universal donor for cell therapy. Journal of the American Heart Association. 7 (23), 010239 (2018).

