Real-Time Quantification of Reactive Oxygen Species in Neutrophils Infected with Meningitic Escherichia Coli
Summary
Escherichia coli is the leading cause of neonatal Gram-negative bacterial meningitis. During the bacterial infection, reactive oxygen species produced by neutrophils play a major bactericidal role. Here we introduce a method to detect the reactive oxygen species in neutrophils in response to meningitis E. coli.
Abstract
Escherichia coli (E. coli) is the most common Gram-negative bacteria causing neonatal meningitis. The occurrence of bacteremia and bacterial penetration through the blood-brain barrier are indispensable steps for the development of E. coli meningitis. Reactive oxygen species (ROS) represent the major bactericidal mechanisms of neutrophils to destroy the invaded pathogens. In this protocol, the time-dependent intracellular ROS production in neutrophils infected with meningitic E. coli was quantified using fluorescent ROS probes detected by a real-time fluorescence microplate reader. This method may also be applied to the assessment of ROS production in mammalian cells during pathogen-host interactions.
Introduction
Neonatal bacterial meningitis is a common pediatric infectious disease. Escherichia coli (E. coli) with a K1 capsule is the most common Gram-negative pathogen causing neonatal bacterial meningitis, accounting for about 80% of the total incidence1,2,3. Despite the advances in the antimicrobial chemotherapy and supportive care, bacterial meningitis is still one of the most devastating conditions with high morbidity and mortality4.
The occurrence of neonatal bacterial meningitis usually begins with bacteremia caused by the entry of pathogenic bacteria into the peripheral circulation from the local lesions of the newborns, followed by penetration through the blood-brain barrier (BBB) into the brain, resulting in the inflammation of the meninges4. The onset of bacteremia depends on the interaction between bacteria and host immune cells including neutrophils and macrophages, etc. Neutrophils, which account for ~50-70% of white blood cells, are the first line of defense against bacterial infections5,6. During the invasion of bacteria, the activated neutrophils are recruited to the infectious sites and release reactive oxygen species (ROS) including the superoxide anion, hydrogen peroxide, hydroxyl radicals, and singlet oxygen7. The ROS undergo redox reactions with the cell membrane, nucleic acid molecules and proteins of the bacteria, resulting in the injury and death of the invading bacteria8. The mitochondria is the main site of ROS production in eukaryotic cells, and various oxidases (e.g., nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex, lipoxygenase system, protein kinase C and cyclooxygenase system) mediate the production of ROS9,10. The real-time measurement of the production of ROS, representing the primary antimicrobial mechanism in neutrophils, is a useful method for studying host defense during the bacteria-host interaction.
In this protocol, the time-dependent ROS production in neutrophils infected with meningitic E. coli was quantified with a fluorescent ROS probe DHE, detected by a real-time fluorescence microplate reader. This method may also be applied to the assessment of ROS production in other mammalian cells during the pathogen-host interaction.
Protocol
Peripheral blood from volunteers applied in this research was approved by the Institutional Review Board of the first Hospital of China Medical University (#2020-2020-237-2).
1. Preparation of reagents and culture medium
- Prepare the red blood cell lysis buffer by adding 8.29 g of NH4Cl, 1 g of KHCO3, 37.2 mg of Na2EDTA into 1 L of double distilled water and adjust the pH to 7.2-7.4. Remove the bacteria by filtration using 0.22 µm filters.
- Prepare experimental culture medium for neutrophils by adding 5% fetal bovine serum to RPMI 1640 medium and store at 4 °C. Equilibrate to room temperature before use.
NOTE: Use the RPMI 1640 medium without phenol red. - Prepare phosphate buffer saline (PBS) by adding 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4·2H2O and 0.2 g of KH2PO4 into 1 L of double distilled water in a 1 L glass flask. Adjust the pH to 7.2-7.4. Autoclave it for 15 min at 121 °C.
- Prepare rifampicin solution by dissolving 0.5 g of rifampicin powder in 10 mL of dimethyl sulfoxide (DMSO) to yield a 50 mg/mL rifampicin solution.
- Prepare LB agar solution by adding 10 g of NaCl, 10 g of tryptone, 5 g of yeast extract and 15 g of agar powder into 1 L of double distilled water and autoclave the mixture. Fill the Petri dishes to half of the volume by pouring warm LB agar solution containing 100 µg/mL rifampicin. Store the cooled solid plate at 4 °C.
- Prepare brain heart infusion (BHI) broth appropriate for bacterial strains by dissolving 37 g of BHI powder into 1 L of double distilled water. Adjust the pH to 7.2 and autoclave it.
- Dissolve the fluorescent probe dihydroethidium (DHE) in DMSO solvent to yield a 10 mM stock solution. Gently mix before use.
NOTE: Aliquot the stock solution immediately into light-proof vials. The shelf life of the stock solution is 6 months at -20 °C.
2. Preparation of E44 bacteria strain
NOTE: E44 is a mutant strain of meningitic E. coli with rifampicin resistance.
- Dip the cryopreserved E44 colony with a sterile pipette tip, inoculate the E44 strain on the LB agar plate containing 100 µg/mL rifampicin by drawing lines. Put the plate upside down in the incubator at 37 °C overnight.
- One day before the experiment, pick one E44 colony from the plate with a sterile pipette tip and put it in 5 mL of BHI broth containing 100 µg/mL rifampicin in a 50 mL flask. Incubate the bacterial culture at 37 °C with 90 rpm for 17 h in an incubation shaker.
3. Isolation of neutrophils from human peripheral blood
- Draw 5 mL of blood sample from volunteers intravenously to the vacuum blood collection tube containing EDTA for anticoagulation.
- Centrifuge the peripheral blood samples at 500 x g for 5 min. The blood samples are divided into three layers by centrifugation, which from bottom to top are the red blood cell (RBC) layer, the white blood cell (WBC) layer and the plasma layer, sequentially.
- Aspirate the white blood cell layer with a pipet to a new tube with 3x RBC lysis buffer. Blend the mixture thoroughly and place at room temperature for 5 min.
- Centrifuge the tube at 500 x g for 5 min. Aspirate the supernatant completely and discard.
- Repeat the lysis procedure with RBC lysis buffer 1-2 times, until the precipitate turns white.
- Wash the cells by resuspending the precipitate with 2 mL of PBS. Then centrifuge at 300 x g for 5 min to let the cells settle down to the bottom of the tube.
- Label the neutrophils with CD16 microbeads by resuspending the sediment with 50 µL of precooled magnetic cell sorting buffer. Then mix with 50 µL of human CD16 microbeads thoroughly. Incubate the mixture at 4 °C for 30 min.
NOTE: Solutions should be pre-cooled to prevent capping of antibodies on the cell surface and non-specific labeling. Most adults have about 4,000 to 10,000 white blood cells per microliter of blood, among which, neutrophils account for approximately 50-70%. By estimate, the counts of total white blood cells in 5 mL of human peripheral blood are usually up to 2-5 x 107. - Wash the cells by adding 2 mL of magnetic cell sorting buffer and centrifuge at 4 °C, 300 x g for 10 min. Discard the supernatant completely and resuspend the precipitate with 500 µL of sorting buffer.
- Assemble the magnetic column and separating shelf. Move the separator to the shelf with the magnetic column and rinse the column with 3 mL of sorting buffer.
- Drop the cell suspension into the column to allow the neutrophils labeled by the magnetic beads to attach to the magnetic column.
- Wash off the non-labeled cells by adding 3 mL of magnetic cell sorting buffer 3 times, making sure that the column reservoir is empty each time.
- Remove the column from the magnetic separator and put it on a 15 mL tube. Add 5 mL of magnetic cell sorting buffer to the column. Push out the magnetic labeled cells using a plunger.
NOTE: To improve the purity of the neutrophils, the sorting steps may be repeated using a new column. - Centrifuge the tube at 300 x g for 5 min, discard the supernatant completely and resuspend the precipitate with 1 mL of culture medium. Determine the cell number with a cell counter and prepare the cells for further experiments.
4. Measurement of ROS
- Centrifuge the isolated neutrophils at 300 x g for 5 min, resuspend the precipitate, and adjust the cell concentration to 2 x 106/mL with culture medium containing 5 µM DHE fluorescence probe.
- Incubate the neutrophils at 37 °C for 30 min to load the DHE probe, and then allocate the cell suspension to a 96-well black polystyrene microplate with 200 µL per well.
- Turn on the microplate reader and open the detection software. Choose opaque 96-wells plate format and determine the reading area.
- Set the fluorescence (Ex/Em = 518/605 nm) in kinetic mode every 5 min for 60 min at 37 °C. Make sure to shake the plate for 3 s before each reading.
- Take out the microplate from the incubator, add the cultured E44 (MOI=100) or phorbol 12-myristate 13-acetate (PMA) (100 ng/mL) to each well containing preloaded neutrophils with 3 replicates. Use PMA as a positive control.
- Place the plate in the microplate reader and start the assay immediately.
Representative Results
Using the protocol outlined in this article, the neutrophils were isolated from human peripheral blood and loaded with fluorescence probe DHE to detect the changes of ROS levels in response to E44 infection. Here, we provide representative data demonstrating the ROS production evoked by E44 strain determined by a microplate reader in real-time. By adding E44 strains at a MOI of 100, the ROS levels increased immediately and showed a continuous upward trend with a time-dependent manner (Figure 1). By adding PMA, a well-known ROS inducer of intracellular ROS in neutrophils, we observed an S-shaped curve that presents a flat curve at the initial stage followed by a significant increase from 20 min to 40 min and finally peaking at 60 min (Figure 1).
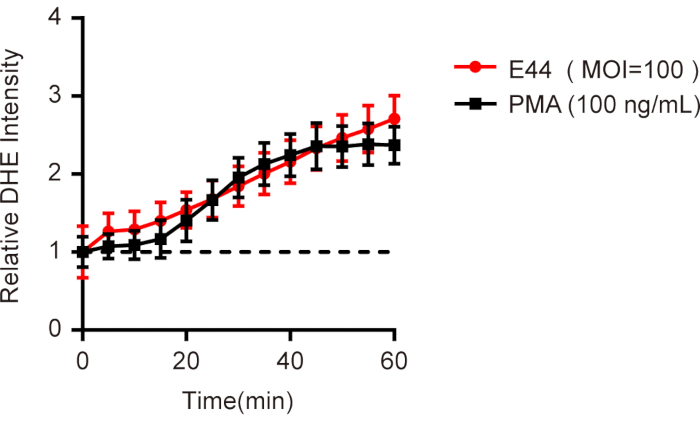
Figure 1. Time-dependent ROS production in neutrophils infected with meningitic E. coli. Neutrophils isolated from human peripheral blood were loaded with DHE dye, an E44 strain (MOI=100) was added, and the mean fluorescence intensity (MFI) was determined immediately with a microplate reader. Neutrophils treated with PMA (100 ng/mL) were used as a positive control. All the data were normalized to the initial value to obtain the relative DHE intensity and presented as mean ± SEM (n = 3). Please click here to view a larger version of this figure.
Discussion
Neutrophils act as the most abundant component of white blood cells in human blood circulation. They are important effector cells in the innate human immune system, which builds the first line of defense against the invasion of pathogens11. The generation of ROS represents one of the major bactericidal mechanisms of neutrophils following phagocytosis11. Recent studies have shown that a net-like structure released by a neutrophil called neutrophil extracellular trap (NET) is also involved in the bacteria killing process6,11,12.
It has been reported that ROS produced by neutrophils induced by the stimulation of pathogenic microorganisms are mainly caused by the activation of NADPH oxidase, which is composed of two membrane binding subunits (gp91phox and p22phox) and three cytosolic subunits (p47phox, p67phox, and p40phox)5. The engulfed bacteria activate a series of kinases inside the neutrophils, such as protein kinase C (PKC), protein kinase A (PKA), and mitogen-activated protein kinase (MAPK), that phosphorylate the cytosolic subunits p47phox of NADPH oxidase. Then a cytosolic trimer composed of p47phox, p67phox, and p40phox translocates to the membrane to combine with the membrane binding subunit gp91phox and p22phox, forming the full NADPH oxidase complex. The assembled NADPH oxidase complex transfers NADPH-derived electrons to molecular O2, generating superoxide anions and activating the bactericidal functions13,14,15. It is also reported that ROS produced by neutrophils may be also associated with the NET formation of neutrophils16,17. Therefore, the detection of ROS production would contribute to the further study of the bactericidal mechanism of neutrophils.
In this protocol, the neutrophils isolated from peripheral blood are preloaded with fluorescence probe DHE and allocated to a 96-well black polystyrene microplate. The ROS intensity is detected by a microplate reader in real-time after meningitic Escherichia coli is added.
The collected blood is anti-coagulated using EDTA or citrate to avoid activation of neutrophils by complement18. As neutrophils are terminally differentiated and have a short life span (about 4-8 hours) in the circulating blood, the isolation steps should be done as soon as possible after blood collection19. Many isolating reagents, such as Ficoll-Hypaque and Percoll, have been used to isolate neutrophils from peripheral blood11,19,20. In this protocol, neutrophils are isolated by CD16 microbead selection after erythrocyte lysis from the peripheral blood. It offers significant improvements in speed, simplicity, and purity. To obtain purer neutrophils, a density gradient centrifugation could be applied before the isolation with CD16 magnetic beads.
A number of recognized fluorescent probes, such as dihydroethidium (DHE), 2', 7'-dichlorodihydrofluorescein diacetate (H2DCF-DA) and dihydrorhodamine 123 (DHR) that pass through the cell membrane freely, can be used for the determination of intracellular ROS by flow cytometry, confocal microscopy or microplate reader21,22,23,24. In addition to the detection of DHE fluorescence probe with microplate reader, flow cytometry and confocal microscopy could also be used to detect the alterations of fluorescence intensity of DHE at the indicated time points to measure the ROS production in neutrophils.
This protocol provides an easier way for the detection of ROS production in a real-time manner and can be used in a variety of scenarios to detect the ROS generation in host mammalian cells infected with pathogenic microorganisms.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31670845, 31870832, 32000811) and the Program of Distinguished Professor of Liaoning Province (LJH2018-35).
Materials
| 15 mL polypropylene conical centrifuge tubes | KIRGEN | KG2611 | |
| 96-well plate | Corning | 3025 | |
| Agar | DINGGUO | DH010-1.1 | |
| Autuomated cell counter | Bio-rad | 508BR03397 | |
| Biological Safety Carbinet | Shanghai Lishen | Hfsafe-1200Lcb2 | |
| Brain heart infusion | BD | 237500 | |
| CD16 Microbeads, human | Miltenyi Biotec | 130-045-701 | |
| Centrifuge | Changsha Xiangyi | TDZ5-WS | |
| Columns | Miltenyi Biotec | 130-042-401 | |
| Dihydroethidium (DHE) | MedChemExpress | 104821-25-2 | |
| Fetal bovine serum | Cellmax | SA211.02 | |
| Incubator | Heraeus | Hera Cell | |
| MACS separation buffer | Miltenyi Biotec | 130-091-221 | |
| Microplate Reader | Molecular Devices | SpectraMax M5 | |
| Phorbol 12-myristate 13-acetate (PMA) | Beyoitme | S1819-1mg | |
| QuadroMACS separation Unit | Miltenyi Biotec | 130-090-976 | |
| Rifampicin | Solarbio | 13292-46-1 | |
| RPMI1640 medium | Sangon Biotech | E600027-0500 | |
| Thermostatic shaker | Shanghai Zhicheng | ZWY-100D | |
| Trypton | OXOID | LP0042 | |
| Yeast extract | OXOID | LP0021 |
References
- Kim, K. S. Acute bacterial meningitis in infants and children. Lancet Infectious Diseases. 10 (1), 11 (2010).
- Woll, C., et al. Epidemiology and Etiology of Invasive Bacterial Infection in Infants </=60 Days Old Treated in Emergency Departments. Journal of Pediatrics. 200, 210-217 (2018).
- Xu, M., et al. Etiology and Clinical Features of Full-Term Neonatal Bacterial Meningitis: A Multicenter Retrospective Cohort Study. Frontiers in Pediatrics. 7, 31 (2019).
- Kim, K. S. Human Meningitis-Associated Escherichia coli. EcoSal Plus. 7 (1), (2016).
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. Journal of Leukocyte Biology. 108 (1), 377-396 (2020).
- Kolaczkowska, E., Kubes, P. Neutrophil recruitment and function in health and inflammation. Nature Reviews: Immunology. 13 (3), 159-175 (2013).
- Winterbourn, C. C., Kettle, A. J., Hampton, M. B. Reactive Oxygen Species and Neutrophil Function. Annual Review of Biochemistry. 85, 765-792 (2016).
- Witko-Sarsat, V., Descamps-Latscha, B., Lesavre, P., Halbwachs-Mecarelli, L. Neutrophils: Molecules, Functions and Pathophysiological Aspects. Laboratory Investigation. 80 (5), 617-653 (2000).
- Zorov, D. B., Juhaszova, M., Sollott, S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiological Reviews. 94 (3), 909-950 (2014).
- Zeng, M. Y., Miralda, I., Armstrong, C. L., Uriarte, S. M., Bagaitkar, J. The roles of NADPH oxidase in modulating neutrophil effector responses. Molecular Oral Microbiology. 34 (2), 27-38 (2019).
- Liew, P. X., Kubes, P. The Neutrophil’s Role During Health and Disease. Physiological Reviews. 99 (2), 1223-1248 (2019).
- Brinkmann, V., et al. Neutrophil Extracellular Traps Kill Bacteria. Science. 303 (5), 1532-1535 (2004).
- Lam, G. Y., Huang, J., Brumell, J. H. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Seminars in Immunopathology. 32 (4), 415-430 (2010).
- Panday, A., Sahoo, M. K., Osorio, D., Batra, S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cellular & Molecular Immunology. 12 (1), 5-23 (2015).
- Nunes, P., Demaurex, N., Dinaue, C. Regulation of the NADPH Oxidase and Associated Ion Fluxes During Phagocytosis. Traffic. 14, 1118-1131 (2013).
- Dahlgren, C., Karlsson, A., Bylund, J. Intracellular Neutrophil Oxidants: From Laboratory Curiosity to Clinical Reality. Journal of Immunology. 202 (11), 3127-3134 (2019).
- Stoiber, W., Obermayer, A., Steinbacher, P., Krautgartner, W. D. The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans. Biomolecules. 5 (2), 702-723 (2015).
- Haynes, A. P., Fletcher, J. neutrophil function test. Clinical Haematology. 3 (4), 871-887 (1990).
- Eichelberger, K. R., Goldman, W. E. Human Neutrophil Isolation and Degranulation Responses to Yersinia pestis Infection. Methods in Molecular Biology. 2010, 197-209 (2019).
- Siano, B., Oh, H., Diamond, S. Neutrophil isolation protocol. Journal of Visualized Experiments. (17), (2008).
- Chen, X., Zhong, Z., Xu, Z., Chen, L., Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radical Research. 44 (6), 587-604 (2010).
- Woolley, J. F., Stanicka, J., Cotter, T. G. Recent advances in reactive oxygen species measurement in biological systems. Trends in Biochemical Sciences. 38 (11), 556-565 (2013).
- Dikalov, S. I., Harrison, D. G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxidants and Redox Signaling. 20 (2), 372-382 (2014).
- Puleston, D. Detection of Mitochondrial Mass, Damage, and Reactive Oxygen Species by Flow Cytometry. Cold Spring Harbor Protocols. 2015 (9), (2015).

