Flow Cytometric Analysis of Lymphocyte Infiltration in Central Nervous System during Experimental Autoimmune Encephalomyelitis
Summary
This manuscript presents a protocol to induce active experimental autoimmune encephalomyelitis (EAE) in mice. A method for the isolation and characterization of the infiltrated lymphocytes in the central nervous system (CNS) is also presented to show how lymphocytes are involved in the development of CNS autoimmune disease.
Abstract
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) caused by the combination of environmental factors and susceptible genetic background. Experimental autoimmune encephalomyelitis (EAE) is a typical disease model of MS widely used for investigating the pathogenesis in which T lymphocytes specific for myelin antigens initiate an inflammatory reaction in CNS. It is very important to assess how lymphocytes in the CNS regulate the development of disease. However, the approach for measuring the quantity and quality of infiltrated lymphocytes in the CNS is very limited due to the difficulties in isolating and detecting infiltrated lymphocytes from the brain. This manuscript presents a protocol for that is useful for the isolation, identification, and characterization of infiltrated lymphocytes from the CNS and will be helpful for our understanding of how lymphocytes are involved in the development of the CNS autoimmune disease.
Introduction
As a chronic demyelinating disease of the CNS, MS affects about 2.5 million people worldwide and lacks curative treatments1. It is also considered an autoimmune disease, in which myelin antigen specific T lymphocytes initiate an inflammatory reaction and lead to demyelination and axonal injury in the CNS2. Experimental autoimmune encephalomyelitis (EAE) has been widely used to investigate pathogenic mechanisms of MS as a classic autoimmune demyelination disease model in CNS3. There are two ways to induce EAE: one is to induce EAE actively by immunizing animals with myelin components, another is adoptive transfer by transferring encephalitogenic T cells into receptor2,4,5. The susceptibilities to EAE are different in different animal strains6. In C57BL/6 mice, myelin oligodendrocyte glycoprotein (MOG) 35–55 challenge induces a monophasic disease with extensive demyelination and inflammation in the CNS, which is frequently used in experiments with gene-targeted mice7.
The generation of myelin-specific reactive T cells is required for the occurrence and development of disease in EAE and is an immunological sign of both EAE and MS. Activated autoreactive T lymphocytes cross the blood brain barrier (BBB) into the healthy CNS and initiate EAE disease. When MOG 35–55 Ag is encountered, these T lymphocytes induce inflammation and the recruitment of effector cells into the CNS, resulting in demyelination and axon destruction8,9. In the EAE model, there is ample evidence that neuroantigen-specific CD4+ T cells can initiate and sustain neuroinflammation and pathology3,10. Depending on the major cytokines produced, CD4+ T lymphocytes have been classified into different subsets: Th1 (characterized by the production of interferon-γ), Th2 (characterized by the production of interleukin 4), and Th17 (characterized by the production of interleukin 17). It is believed that activation of Th1 and Th17 cells contribute to the induction, maintenance, and regulation of inflammatory demyelination in EAE and MS by secreting effector cytokines IFN-γ and IL-17, which are capable of activating macrophages and recruiting neutrophils to the inflammatory sites to accelerate the lesions11.
Because autoreactive T cells cross the BBB into the CNS and induce the development of disease in MS and EAE, it is very important to analyze T cells in the CNS. However, there are very few established protocols for the isolation of lymphocytes from the CNS12. Therefore, a method optimized for isolating mononuclear cells from the brain and analyzing T lymphocytes with markers CD45, CD11b, CD3, CD4, INF-g, and IL-17 for flow cytometry was developed. The method uses MOG35–55 adjuvant Mycobacterium tuberculosis H37 Ra and Pertussis Toxin Working Solution (PTX) to induce an active immunization model of EAE in mice. Then, mechanical separation and density gradient centrifugation methods are used for the isolation of CNS mononuclear cells. Finally, an optimized flow cytometry gating strategy is used to identify T lymphocytes and subsets from the brain by staining multiple markers.
Protocol
All methods described here have been approved by the animal committee of the School of Basic Medical Sciences, Shanghai Jiao Tong University.
1. Preparation of the materials
- Use the MEVGWYRSPFSRVVHLYRNGK sequence of MOG35–55 to obtain the lyophilized peptide from commercial sources. Ensure that the purity of the peptide is >95%. Prepare 10 mg/mL MOG stock solution in phosphate-buffered saline (PBS) and store at -20 °C.
- Prepare a 4 mg/mL stock solution of M. tuberculosis H37 Ra by putting one 100 mg tube of M. tuberculosis H37 Ra into 25 mL of Complete Freund's Adjuvant (CFA) and mixing. Store the stock solution at -20 °C.
- Prepare 1 ng/μL Pertussis Toxin Working Solution (PTX) by adding 50 μg of PTX into 50 mL of PBS. Store the working solution at -20 °C.
- Store all antibodies (i.e., FITC anti-mouse CD3, PE/Cy7 anti-mouse CD4, PerCP/Cy5.5 anti-mouse CD11b, Alexa Fluor700 anti-mouse CD45.2, PE anti-mouse IL-17A, and APC anti-mouse IFN-γ) at 4 °C.
- Make the Flow Cytometry Staining (FCS) Buffer by adding 2 mM ethylene diamine tetraacetic acid (EDTA) and 1% fetal bovine serum (FBS) into 500 mL of PBS.
2. Housing of C57BL/6 mice
- Use female C57BL/6 mice at 8–12 weeks of age.
- Acclimate C57BL/6J mice for at least 7 days prior to the injection.
- House mice in an animal facility under pathogen-free conditions at constant temperature and humidity in a 12 h light/dark cycle and provide free access to water and standard pellet food.
3. Immunization of C57BL/6 mice
- Leave all stock solutions for 15 min at room temperature (RT) to ensure complete rehydration.
- Dilute 300 μL of MOG-peptide stock solution with 700 μL of PBS for preparing 3 mg/mL work solution.
- Put 1 mL of M. tuberculosis H37 Ra stock solution and 1 mL of MOG35–55 peptides working solution into separate 10 mL syringes, then use a four-way stop cock to emulsify for at least 10 min. Ensure complete emulsification before injection.
- Anesthetize mice at the peak of EAE with an intraperitonial injection of 1% sodium pentobarbital (50 mg/kg).
- With a 1 mL syringe, subcutaneously inject mice with 100 μL of a MOG 35–55/CFA emulsion (300 µg/200 μL) at two sites, both at the back near the neck. Subcutaneously inject control mice with 200 μL of PBS.
- On the same day (day 0) and on day 2 post immunization (PI), Intraperitoneally inject mice with 200 μL of 1 ng/μL PTX working solution. Intraperitoneally inject control mice with 200 μL of PBS.
- Transfer the mice to their home cage with a warming pad.
- Examine and grade all mice every day after the injection in a blinded manner for the neurological signs shown in Table 111,13. Euthanize the animals if the scores are worse than grade 4.
- Record the weight changes during the disease course. This is a valuable additional measure for disease activity in the EAE model11,13.
- Add the first day of clinical signs for individual mice and divide by the number of mice in the group; the result is the onset. Add the first day of the maximum EAE score for individual mice and divide by the number of mice in the group; the result is the peak.
4. Single-cell suspension preparation from brain
- Dilute density gradient medium in 9:1 ratio with PBS in a 15 mL conical tube to yield a final 100% solution.
- Anesthetize mice at the peak of EAE with an intraperitonial injection of 1% sodium pentobarbital (50 mg/kg) and perfuse intracardially with 20 mL of sterile ice-cold PBS. Achieve this by slowly and steadily injecting PBS into the left ventricle of the heart using a 20 mL syringe and opening the right atrium.
- Cut the cranium carefully from the nose to the neck, then remove the brain from the cranial box into 10 mL of RPMI in 50 mL conical tubes. Mix well to remove adherent red blood cells. Then remove the medium by aspiration and add 10 mL of RPMI.
- Place the brains and medium in a 100 mm dish. Finely chop with a razor blade.
- Transfer 6 mL from the dish to an ice-cold 7 mL sintered glass homogenizer with a clean pipette. Avoid leaving large quantities of tissue in the pipette. A small amount is unavoidable.
- Grind the brain using the "loose" plunger of the pestle first, then use the "tight" plunger until the suspension is homogeneous, and pour into a prechilled 15 mL conical tube and keep on ice.
- After all the samples are homogenized, estimate the volume. Adjust the volume with RPMI to 7 mL. Then place 3 mL of ice-cold 100% basement membrane matrix in a chilled 15 mL conical centrifuge tube and add 7 mL of the brain homogenate to yield a final 30% density gradient medium. Mix by inversion a couple of times. Do not vortex.
- To ensure a sharp interface, carefully and slowly add 1 mL of 70% underlay density gradient medium in RPMI with a 3 mL pipette.
- Centrifuge at 800 x g for only 20 min at 4 °C. Set the acceleration to 1 and deceleration to 0. After centrifugation, aspirate almost all of the top phase, being careful to completely remove the myelin at the top (Figure 1).
- Remove the interface into a new 15 centrifuge tube. Adjust the volume to 10 mL with RPMI.
- Centrifuge at 500 x g for 10 min. After centrifugation, aspirate the supernatant. Resuspend the pellet in ~200 μL of flow cytometry staining (FSC) buffer. The pellets are then ready to stain for FACS.
5. Flow cytometric analysis of single cells from brain
- Use a hemocytometer and microscope to count the cells. Add 10 μL of the cells to 10 μL of trypan blue, mix well, and place 10 μL on a hemocytometer to count the cells. Then calculate the number of live cells per microliter under an inverted microscope (e.g., Olympus Inverted microscope).
- Aliquot approximately 2 x 106 of cells in RPMI into a single well of a 96 well plate.
- Add 500x cell stimulation cocktail plus protein transport inhibitors to the wells.
- Incubate the plate in the incubator at 37 °C for 4 h.
- Centrifuge the cells at 400 x g for 5 min at RT. Discard the supernatant and resuspend the cells in 100 μL of FCS Buffer.
- Preincubate the cells with anti-mouse CD16/CD32 Fc block (1:33) for 10 min at 4 °C before staining to block nonspecific Fc-mediated interactions.
- Stain cell surface markers without washing. Add anti-mouse CD45.2 (1:200), anti-mouse CD11b (1:200), anti-mouse CD3 (1:200), and anti-mouse CD4 (1:200) antibodies.
NOTE: To determine positive and negative gates, a fluorescence minus one (FMO) for each color and an isotype control antibody should be stained. - Incubate the plate for at least 30 min at 4 °C or on ice. Protect from light.
- Wash the cells by adding FCS Buffer. Use 200 μL/well for microtiter plates. Centrifuge at 400 x g for 5 min at RT. Discard the supernatant.
- Add 200 μL of intracellular (IC) fixation buffer to each well to fix the cells. Ensure the cells are fully resuspended in the solution.
- Incubate 30–60 min at RT. Protect from light.
- Centrifuge the samples at 400 x g at RT for 5 min. Discard the supernatant.
- Add 200 μL of 1x permeabilization buffer to each well and centrifuge the samples at 400 x g at RT for 5 min. Discard the supernatant.
- Resuspend the pellet in residual volume and adjust volume to about 100 μL with 1x permeabilization buffer.
- Add anti-mouse IL-17A (1:200) and anti-mouse IFN-g (1:200) antibodies for detection of intracellular antigens to cells.
- Incubate for at least 30 min at 4 °C. Protect from light.
- Add 100 μL of 1x permeabilization buffer to each well and centrifuge the samples at 400 x g at RT for 5 min. Discard the supernatant.
- Resuspend the stained cells in 100 μL of flow cytometry staining buffer.
- Analyze by flow cytometry.
NOTE: The laser and compensation settings on the flow cytometer are adjusted, the samples are placed onto the cytometer, and all events are recorded as per the manufacturer’s recommendations.
6. Data analysis
- Gate singlets using FSC-A vs. FSC-H and SSC-A vs. SSC-H.
- Gate live cells using FSC-A and SSC-A based on size.
- Next, identify the leukocytes, excluding the monocytes, by gating on CD45+ CD11b– cells.
- Then, identify the CD4 T lymphocytes by gating on CD3+CD4+ cells.
- Lastly, identify the Th1 and Th17 subsets by gating on IFN-γ+ cells and IL-17+ cells separately, and determine the positive and negative populations using isotype controls and FMO.
Representative Results
After immunization of C57BL/6 mice, all mice were weighed, examined, and graded daily for neurological signs. The representative clinical course of EAE should result in a disease curve as presented in Figure 2A and a change of body weight in the mouse as presented in Figure 2B. C57BL/6 mice immunized with MOG35-55 usually started to develop disease symptoms around day 10–12 and achieved the peak of disease around day 15–21 after active immunization (Figure 2A). Weight change was a valuable indicator in the EAE model. Before the onset of disease, the body weight of immunized mice gradually increased, then typically decreased correlating to the increasing disease symptoms. At the peak of EAE, mice also showed the lowest body weight (Figure 2B). Then body weight recovered slightly as clinical symptoms decreased. However, the mice usually did not fully recover. C57BL/6 mice developed a monophasic chronic disease pathology upon MOG35–55 challenge (Figure 2A).
The clinical severity of EAE is directly associated with autoreactive T cell activation14. Neuroantigen-specific CD4+ T cells are capable of initiating and sustaining neuroinflammation and pathology in EAE3. The typical characteristics of CD4+T cells in the peak of EAE are shown in Figure 3. Further, the proportion of Th1 and Th17 subsets in encephalitogenic cells, which are the major pathogenic cells mediating EAE, was analyzed by flow cytometry. Following the separation of a single-cell suspension from brain, the cells were stained with CD45, CD11b, CD3, CD4, IFN-γ, and IL-17 antibodies, which are expressed by T lymphocytes. FSC-A vs. FSC-H and SSC-A vs. SSC-H were used to gate singlets (Figure 3A, B), then FSC-A vs. SSC-A were used to gate live cells based on size and granularity (Figure 3C). After, CD45+ CD11b– cells were gated to identify leukocytes excluding monocytes (Figure 3D). Then, the gate was set on CD3+CD4+ to identify CD4+ T lymphocytes (Figure 3E). Finally, IFN-γ and IL-17 were used to identify Th1 and Th17 subsets and assess the functional effect according to effector cytokines (Figure 3F). According to the clinical severity of disease, representative results showed that IFN-γ–producing Th1 and IL-17–producing Th17 cells significantly increased in the encephalitogenic cells from EAE mice.
| Grade | Clinical sign |
| 0 | no disease |
| 1 | decreased tail tone or slightly clumsy gait |
| 2 | tail atony and moderately clumsy gait and/or poor righting ability |
| 3 | limb weakness |
| 4 | limb paralysis |
| 5 | moribund state. |
Table 1: Clinical scoring system. C57BL/6 mice were immunized with the MOG35–55 peptide. Then, neurological signs were recorded. A 5-point scoring system was used to assess the severity of EAE.
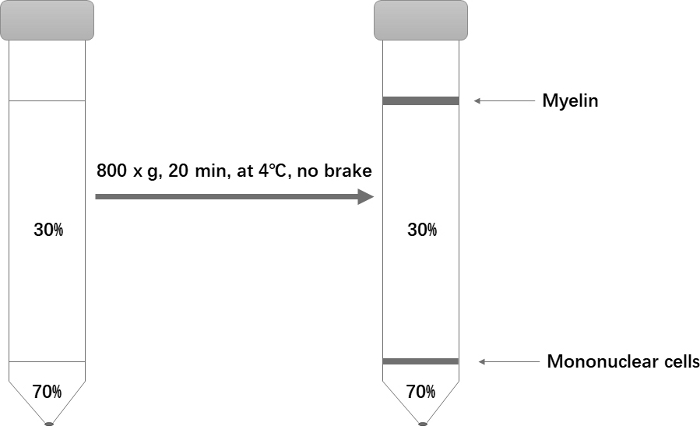
Figure 1: Schematic of the Percoll gradient setup for isolation of mononuclear cells. Please click here to view a larger version of this figure.
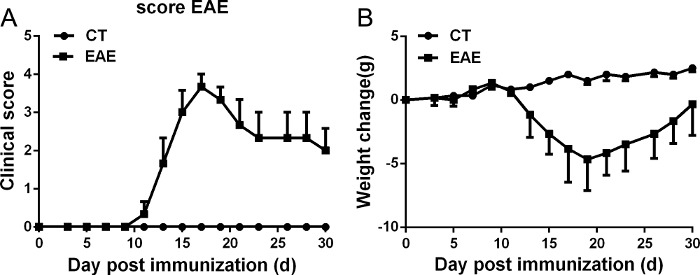
Figure 2: Representative course of EAE. EAE was induced in C57BL/6 mice by injection of MOG35–55 as described in the protocol. The clinical score (A) and change of body weight (B) were determined in these mice. Data are presented as mean ± SEM; n = 8 for each group. Please click here to view a larger version of this figure.
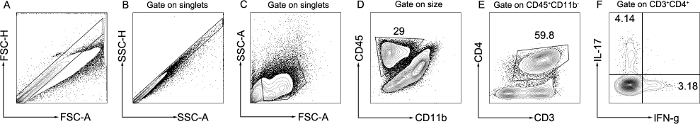
Figure 3: Representative flow cytometry analysis of lymphocytes in brain. A single-cell suspension was isolated from the brain in the peak of EAE. The gating strategy of T lymphocytes is shown. Singlets were gated as FSC-A vs. FSC-H and SSC-A vs. SSC-H (A,B). Live cells were gated as FSC-A vs. SSC-A (C). Leukocytes excluding monocytes were gated as CD45+ CD11b– (D). CD4+ T lymphocytes were gated as CD3+CD4+ (E). Th1 and Th17 subsets were gated as IFN-γ+ and IL-17+ (F). Please click here to view a larger version of this figure.
Discussion
This study presents a protocol to induce and monitor EAE using MOG35-55 in C57BL/6 mice, which are considered a typical neuroimmunological experimental animal model of MS. EAE can be induced varying the mice strains or the type of protein used for induction according to the purpose of the study. For example, using PLP139–151 peptide in SJL mice can induce a relapsing-remitting EAE disease course that is especially well-suited for assessing therapeutic effects on relapses15. The experimental procedure outlined here can be also applied to other EAE protocols7. In this model, C57BL/6 mice are immunized with MOG35–55 peptide and develop a monophasic disease. A 5-point scoring system is used to assess the severity of EAE. Although several scoring systems ranging from 0–3 points or 0–10 points are employed to score disease severity7,16,17, these results show that a 5-point scoring system is capable of determining statistically significant differences in disease scores between groups and other EAE scoring systems do not lead to obvious improvement.
EAE severity is generally evaluated by an EAE clinical score taking into account the severity of neurological dysfunction11,13. To ensure the comparability of the experiment for all mice, it is important to keep them under the same conditions, including changes of cage, administration of food and water, and especially mouse housing conditions. In addition, cross-immunization should be also performed to avoid cage-specific phenomena induced by the investigator.
This study provides a method to separate mononuclear cells from the CNS that is suitable for FACS analysis or functional study. To ensure that the blood is removed from the CNS tissue, the mice should be perfused prior to dissociating the tissue. The purification of the mononuclear cells on a density gradient centrifugation is a key step in the isolation. To ensure a separation effect, the acceleration and deceleration of the centrifuge should be set to 1 and 0, respectively. Using this method, the single cell yields are usually low from normal brain, but higher from diseased brain with EAE. Representative results show that there is an obvious increase in CD3+CD4+ T lymphocytes, especially the IFN-γ producing cells and IL-17 producing cells, which are considered to contribute to the worsened disease.
There are some limitations of this protocol. The EAE model induced with MOG35–55 shows mainly a CD4+ T cell-driven immunological response. If the role of CD8+ T cells and B cells needs to be analyzed, alternative protocols should be considered. As a CNS inflammatory disease, a severe pathological phenotype is also found in the spinal cord in the EAE model. However, due to the presence of large amounts of myelin, it is difficult to get enough single cells from the spinal cord for FACS analysis. In that case, using immunohistochemistry or immunofluorescence to analyze spinal cord tissue is needed. There are also researchers that put the brain and spinal cord together to separate mononuclear cells for FACS analysis12. This protocol separates single cells from the brain for FACS analysis and spinal cord tissue for immunohistochemistry and immunofluorescence analysis.
The importance of T lymphocytes in immune regulation of MS and EAE has received more and more attention recently. Much of the published literature focuses on spleen and lymph nodes11; however, lymphocytes are found throughout the CNS of EAE mice and, thus, the characteristic analysis of T lymphocytes in the CNS is necessary. Immunohistological staining of sections can identify infiltrating cells in the CNS. However, phenotypic and functional analysis is limited. Following isolation of the immune cells from the CNS of normal or diseased mice, the analysis of more detailed phenotypes becomes possible. With this method, T lymphocytes in the brain can be studied on a cell-by-cell basis, and the expression of different surface markers, cytokines, chemokines, and transcription factors (e.g., intracellular proteins) can be analyzed better. The protocol will be useful for future studies to assess the phenotype and function of T lymphocytes in the brain during the course of MS and EAE.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was supported by National Natural Science Foundation of China grant (31570921 to ZJ, 81571533 to LS), Shanghai Municipal Commission of Health, and Family Planning (201540206 to ZJ), Ruijin Hospital North research grant (2017ZX01 to ZJ).
Materials
| Alexa Fluor700 anti-mouse CD45.2 | eBioscience | 56-0454-82 | |
| Anti-Mouse CD16/CD32 Fc block | BioLegend | 101302 | |
| APC anti-mouse IFN-g | eBioscience | 17-7311-82 | |
| BD LSRFortessa X-20 | BD | ||
| Dounce homogenizer | Wheaton | 353107542 | |
| eBioscience Cell Stimulation Cocktail (plus protein transport inhibitors) (500X) | eBioscience | 00-4975-03 | |
| eBioscience Intracellular Fixation & Permeabilization Buffer Set | eBioscience | 88-8824-00 | |
| FITC anti-mouse CD3 | BioLegend | 100203 | |
| FITC Rat IgG2b, κ Isotype Ctrl Antibody | BioLegend | 400605 | |
| Freund's Adjuvant Complete (CFA) | Sigma-Aldrich | F5881 | |
| Mouse IgG2a kappa Isotype Control (eBM2a), Alexa Fluor 700, eBioscience | eBioscience | 56-4724-80 | |
| Mycobacterium tuberculosis H37 Ra | Difco Laboratories | 231141 | |
| PE anti-mouse IL-17A | eBioscience | 12-7177-81 | |
| PE/Cy7 anti-mouse CD4 | BioLegend | 100422 | |
| PE/Cy7 Rat IgG2b, κ Isotype Ctrl Antibody | BioLegend | 400617 | |
| Percoll | GE | 17-0891-01 | |
| PerCP/Cy5.5 anti-mouse CD11b | BioLegend | 101228 | |
| PerCP/Cy5.5 Rat IgG2b, κ Isotype Ctrl Antibody | BioLegend | 400631 | |
| pertussis toxin (PTX) | Sigma-Aldrich | P-2980 | |
| Rat IgG1 kappa Isotype Control (eBRG1), APC, eBioscience | eBioscience | 17-4301-82 | |
| Rat IgG2a kappa Isotype Control (eBR2a), PE, eBioscience | eBioscience | 12-4321-80 | |
| Rat MOG35–55 peptides | Biosynth International | MEVGWYRSPFSRVVHLYRNGK |
References
- Milo, R., Kahana, E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmunity Reviews. 9, 387-394 (2010).
- Gold, R., Linington, C., Lassmann, H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain : A Journal of Neurology. 129, 1953-1971 (2006).
- Simmons, S. B., Pierson, E. R., Lee, S. Y., Goverman, J. M. Modeling the heterogeneity of multiple sclerosis in animals. Trends in Immunology. 34 (8), 410-422 (2013).
- Lassmann, H., Wisniewski, H. M. Chronic relapsing experimental allergic encephalomyelitis: clinicopathological comparison with multiple sclerosis. Archives of Neurology. 36, 490-497 (1979).
- Bernard, C. C., Leydon, J., Mackay, I. R. T cell necessity in the pathogenesis of experimental autoimmune encephalomyelitis in mice. European Journal of Immunology. 6, 655-660 (1976).
- Yasuda, T., Tsumita, T., Nagai, Y., Mitsuzawa, E., Ohtani, S. Experimental allergic encephalomyelitis (EAE) in mice. I. Induction of EAE with mouse spinal cord homogenate and myelin basic protein. Japanese Journal of Experimental Medicine. 45, 423-427 (1975).
- Mendel, I., Kerlero de Rosbo, N., Ben-Nun, A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. European Journal of Immunology. 25, 1951-1959 (1995).
- Bramow, S., et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 133, 2983-2998 (2010).
- Sospedra, M., Martin, R. Immunology of multiple sclerosis. Annual Reviews in Immunology. 23, 683-747 (2005).
- McGinley, A. M., Edwards, S. C., Raverdeau, M., Mills, K. H. G. Th17 cells, gammadelta T cells and their interplay in EAE and multiple sclerosis. Journal of Autoimmunity. 20 (14), 3394 (2018).
- Ji, Z., et al. Thiamine deficiency promotes T cell infiltration in experimental autoimmune encephalomyelitis: the involvement of CCL2. Journal of Immunology. 193, 2157-2167 (2014).
- Manglani, M., Gossa, S., McGavern, D. B. Leukocyte Isolation from Brain, Spinal Cord, and Meninges for Flow Cytometric Analysis. Current Protocols in Immunology. 121, 44 (2018).
- Ji, Z., et al. Obesity promotes EAE through IL-6 and MCP-1-mediated T cells infiltration. Frontiers in Immunology. 10, 1881 (2019).
- Reiseter, B. S., Miller, G. T., Happ, M. P., Kasaian, M. T. Treatment of murine experimental autoimmune encephalomyelitis with a myelin basic protein peptide analog alters the cellular composition of leukocytes infiltrating the cerebrospinal fluid. Journal of Neuroimmunology. 91, 156-170 (1998).
- Bittner, S., Afzali, A. M., Wiendl, H., Meuth, S. G. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. Journal of Visualized Experiments. (86), e51275 (2014).
- Miller, S. D., Karpus, W. J. Experimental autoimmune encephalomyelitis in the mouse. Current Protocols in Immunology. , 11 (2007).
- Tietz, S. M., Engelhardt, B. Visualizing Impairment of the Endothelial and Glial Barriers of the Neurovascular Unit during Experimental Autoimmune Encephalomyelitis In Vivo. Journal of Visualized Experiments. (145), e59249 (2019).

