How to Obtain Reliable Visual Event-related Potentials in Newborns
Summary
Several important points for obtaining high-quality reliable visual evoked potentials (VEPs) in newborns while minimizing variability and the risk of misleading prognoses are presented.
Abstract
The present study discusses the characteristics of visual event-related potentials (VEPs) and outlines methodological steps for obtaining reliable measurements in newborns. Obtaining high-quality, reliable VEPs is crucial for the early detection of abnormal development of the central nervous system in at-risk newborns, and for implementing successful early interventions. Recommendations are based on a previous study which showed that when post-conceptional age, polysomnography-identified sleep stages, and light-emitting diodes (LEDs) googles as the luminous source are controlled, no more than 4 repetitions of VEP averages are required to obtain replicable recordings, variability decreases, and reliable VEPs can be obtained. By controlling for these sources of variability and using statistical analyses, we were able to clearly and reliably identify the amplitude and latency of three main components (NII, PII and NIII) present in 100% of newborns (n = 20) during active sleep. Recording VEPs during awake states, quiet sleep and transitional sleep is not recommended because VEP morphology may differ significantly from one average to the next, leading to the risk of misleading clinical prognoses. Moreover, it is easier to obtain VEPs during active sleep because this state can be clearly and reliably identified at this stage of development, sleep cycles are short enough to allow measurements to be taken in a reasonable time, and the method does not require new o expensive equipment.
Introduction
Early detection of abnormal development of the central nervous system in at-risk newborns is crucial for successful early interventions1,2. Visual event-related potentials (VEPs) provide a useful means of evaluating visual cortical status because they do not require patient cooperation, which is not possible in the first month of life, are objective, and are sensitive to structural and functional brain damage3,4.
Though, some studies of newborns have shown that normal visual-evoked responses indicate adequate neural maturation of the cerebral cortex4,5, and that this has often been studied in newborns to assess neurodevelopment and identify abnormal development of the visual pathways4,5, the clinical use of VEPs has been limited by the variability observed in their morphology4,5,6,7. Therefore, it is important to obtain better, more reliable characterizations of VEPs in newborns.
One cause of the variability in VEP morphology is that earlier studies have mixed preterm and older babies (over one month)8,9,10. However, the most important source is the lack of attention paid to the infants' behavioral state while recording VEPs; namely, awake, quiet (QS), active (AS), or transitional sleep. QS and AS have either not been analyzed separately5,11,12, or studies have relied exclusively on behavioral observation without using polysomnography to identify states7,8. Tracé alternant, which consists in bursts of high amplitude slow activity alternating with inter-burst intervals of minimal amplitudes is present in QS, but has not been taken into account when averaging VEPs. Some studies with newborns have measured VEPs by recording during wakefulness13,14, but at this stage of development waking periods are brief and newborns are usually crying or moving, which makes it difficult to obtain high quality, reliable recordings.
Few studies have used light-emitting diodes (LEDs) googles6,9 to elicit VEPs, though this light source generates more consistent recordings than the usual strobe flashes of white light11,14,15, which are less reliable. Obtaining replicable VEPs in the same newborn is indispensable for clinical use4, but another cause of variability is the low reproducibility of VEP morphology, likely due to the lack of control of physiological states and of the stimuli used to elicit VEPs. Given these conditions, the high variability of VEP morphology is hardly surprising.
A previous study conducted with 20 healthy full-term newborns that considered several sources of variability: post-conceptional age, polysomnographically-identified sleep states, LED googles to elicit VEPs, and measures of reproducibility between two VEP averages found that a clearer, more reliable VEP morphology can be obtained during active sleep. During this sleep stage all infants generated clear VEPs with higher correlations between two averages than in QS. Also, fewer VEP averages were required to obtain reproducibility16.
Given the clinical usefulness of VEP studies to assess, as early as possible, the integrity of visual pathways, this study proposes a series of methodological steps designed to obtain reliable VEPs in preterm and older newborns, using LED goggles during AS unambiguously defined by simultaneous polysomnography.
Protocol
1. Preparation of the Newborns
NOTE: The procedure followed is innocuous and painless, so there are no counter-indications for evaluating full-term and preterm newborns, once they are clinically stable.
- Ensure two and half hour fasting and wakefulness before beginning the study, in neonates older than 40 weeks of postconceptional age.
- Make sure that the baby's head be washed with neutral soap the day before the study. Thus, his/her hair will be clean and dry. Do not apply conditioners.
- Allow the mother to start feeding the newborn 30 min before beginning the study. Allow him/her to burp and start the sleep wrapped in sheets. This will ensure that he/she sleeps easily and spontaneously.
- Wash hands carefully before handling the neonate.
- Use sanitary masks.
- Gently wipe the scalp of the newborn with a cotton ball or gauze soaked in alcohol to remove residual dirt and superficial grease, before the neonate falls asleep.
- Measure the distance between nasion and inion, and between both pre-auricular pits. Calculate 10% and 20% to ensure proper placement of the cranial electrodes according to the international 10-20 system of electrode placement.
- Cover the newborn's entire head with a tubular elastic mesh for the correct attachment of the electroencephalography (EEG) and VEP electrodes. Leave the face fully free and exposed, as shown in Figure 1.
- Mark on the mesh the location of the surface electrodes.
- Use a swab to perfectly separate the newborn's hair at the sites where each electrode will be placed, and lightly rub the skin with abrasive gel for neurophysiological studies.
NOTE: Reschedule the study if the neonate takes more than 2 h to fall asleep.
2. Placement of the Surface Electrodes for EEG and VEP Sleep Recording
NOTE: Before beginning, set the values of the instrument's frequency filters using the specifications in Table 1. It is advisable to connect all electrodes to the EEG and VEP instruments before placing them on the newborn.
- Place the elastic band sensor on the baby's chest to record thoracic respiratory expansion.
- Place the individual surface disc electrodes (standard silver-silver chloride, or gold disc electrodes) with conductive paste through the mesh to fix them in the cranial locations established by the international 10-20 EEG system, adapted for newborns.
- Locate the cranial electrodes for EEG at leads F3, F4, C3, C4, O1 and O2, or at least C3 and C4, referred to linked earlobes, to identify the stages of neonatal sleep.
- Fix the surface disc electrodes to the skin with medical adhesive tape. To record ocular movements (EOG), place one electrode 1 cm above the external canthus of the left eye and place another 1 cm below the external canthus of the right eye, also referred to linked earlobes.
- Similarly, attach the electrodes for surface electromyogram recording (EMG) on both sides of the chin, referenced against each other.
- Use two channels of the VEP equipment with the following leads: Oz (-) vs. Fz (+), and Oz (-) vs. A1 (+); the ground electrode is to be placed on the right mastoid.
- Set the analysis time for VEP registration in 600 ms.
NOTE: Table 1 shows the filter settings used for recording sleep EEGs and VEPs. - Do not begin VEP recording until impedance values are below 5 kΩ.
3. Sleep Recording
NOTE: VEPs are obtained while the newborn sleeps in hospital crib; the sleep stages are monitored simultaneously by polysomnography17,18.
- Prolong the EEG recording for 60−90 min or until AS is identified, to evaluate active (AS) and quiet sleep (QS) in newborns.
- Begin EEG recording while carefully observing the characteristics of neonatal sleep, to identify the active sleep stage, during which VEPs will be recorded.
- Identify neonatal sleep stages according to the criteria summarized in Table 2.
4. VEP Recording
NOTE: VEPs are registered according to established standards19,20.
- Allow one minute of EEG recording without visual stimulation when the neonate begins well-defined active sleep.
- Apply monocular light stimulation through handheld goggles with a LED matrix held manually 2 cm directly above each newborn's eyes.
- Observe if the infant has his eyes closed during VEP registration in AS and note if this does not occur.
- Begin the averaging of the VEPs in the equipment, presenting 20 to 40 luminous stimuli whose corresponding recordings are averaged to obtain an average curve or evoked response.
- Observe the reproducibility of the recorded averages. At least two reproducible evoked potentials are recommended.
- Visually recognize PII component of the VEPs during recording, since this peak is considered typical of neonatal VEPs. Identify the PII component as the maximal positive peak between 120 and 300 ms, preceded by a negative wave (NII) and followed by a maximal negativity between 200 and 400 ms, also called NIII.
- Stop the averaging of the VEPs if the newborn moves excessively, wakes up, or changes to another sleep stage, distinct from AS. Renew recording once the AS stage reestablishes.
NOTE: This point is critical, because VEPs obtained during QS or transitional sleep are less reliable than in AS. - Finish registration after 2 averages with reproducible VEP are attained, or when 6 averages occur without a recognizable VEP. In the latter case, consider the result an absence of a replicable response.
5. Review and analysis of VEPs
NOTE: Figure 2 shows the main components of neonatal VEPs and their measurements.
- Evaluate the reproducibility of the VEPs by similar appearance and measurements between the two averaged curves.
NOTE: Some VEP recording systems offer a correlation measure between two averages. - Measure the absolute latencies of the NII, PII and NIII waves using the device's cursors. Absolute latency is the time in ms elapsed from the onset of stimulation to the maximal or minimal peak of each component.
- Calculate the interpeak latencies in ms, including the differences between the absolute PII-NII, NII-NIII and PII-NIII latencies.
- Measure peak-to-peak amplitudes in µV, for the NII-PII and PII-NIII components.
- Compare the latency and amplitude values obtained to the normal, or expected, values estimated for a population of healthy, similar-age newborns.
Representative Results
To detect adequate maturation in the function of the visual pathway it is essential to obtain the PII component of the VEP, which can be seen in both term and preterm infants. The simultaneous recording of VEPs with polysomnography during AS makes it possible to obtain typical VEPs.
Reliable VEP studies require obtaining reproducible average waveforms that will be indispensable for clinical use. Figure 2 illustrates, in a healthy full-term newborn, a clear positivity around 200 ms, which is compatible with the PII component. NII, which corresponds to a preceding, negative small potential, is evident at about 130 ms. The NIII component follows PII as a negativity of approximately 300 ms.
Figure 3A shows three epochs of sleep EEGs, with the typical aspects of AS, QS and tracé alternant. Figure 3B shows the following: a typical VEP waveform with a clear PII in a full-term newborn; an immature response observed in preterm newborns that is normal at this age; and a non-replicable waveform, with waves that do not reproduce exactly the shape of the previous average, thus making it impossible to reliably measure the true latency or amplitude of the components. These VEPs were obtained in a 36 weeks preterm newborn with periventricular leukomalacia.
The application of these procedures allows obtaining reproducible VEPs, but the waveform may vary, according to the age of the newborn and the presence of risk factors. For example, in premature neonates, the form of the VEP can show an immature appearance with inverse polarity and maximum negative amplitudes, but these will change as the baby approaches the full-term age. It is important to identify these normal differences and their relation to the newborn's age, since the true condition of abnormality is given by the absence of reproducible responses or the interhemispheric asymmetry of the potentials obtained by monocular stimulation.
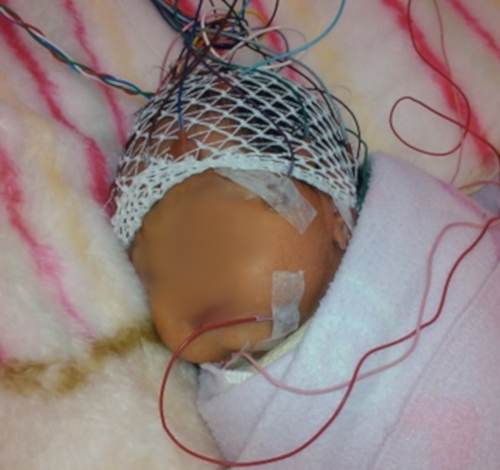
Figure 1: Final placement of the surface electrodes to perform EEG and VEP recording in neonates. Observe the location of the cranial electrodes below the elastic mesh, and the immobilization of the newborns by wrapping them in blankets. Please click here to view a larger version of this figure.
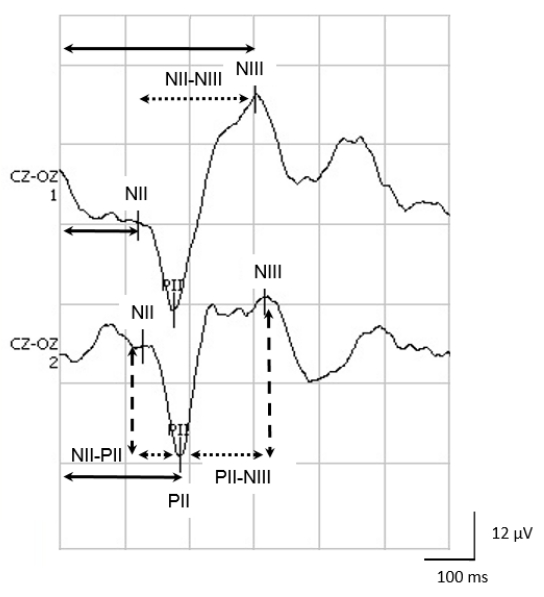
Figure 2: The NII, PII and NIII components of neonatal VEPs. Observe the measurements of the absolute latencies for NII, PII and NIII (solid arrows); the inter-peak intervals for NII-PII, PII-NIII and NII-NIII (dotted arrows); and the amplitude of the different components (dashed arrows). Please click here to view a larger version of this figure.
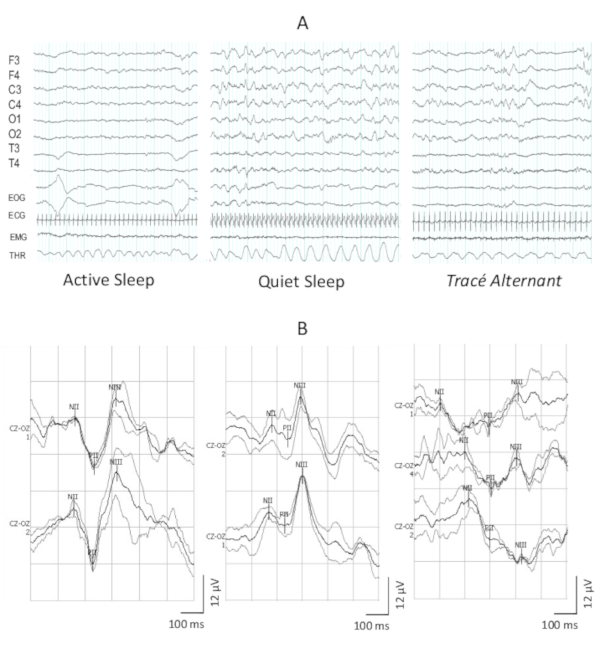
Figure 3: Illustration of the EEG tracings in the sleep stages and the VEPs obtained. (A) Three 30 s periods of neonatal EEG with the main features of the stages of AS, QS and tracé alternant. (B) Three examples of VEPs waveform morphology in neonates, the first with 40 weeks of post-concepcional age, the second with 35 weeks, both are healthy newborns. Note in the second example the polarity inversion. The third is an example of a non-reproducible response, obtained in a 36 weeks newborn with periventricular leukomalacia. Please click here to view a larger version of this figure.
| EEG channels | Sensitivity | Low-frequency filter | High-frequency filter | Display time | Notch filter (60 Hz) | Sampling rate |
| Scalp leads (according to the international 10-20 system) | 0.5 Hz | 30 Hz | 30 s | ON | 200 Hz | |
| Electrooculogram (EOG) | 0.5 Hz | 30 Hz | 30 s | ON | 200 Hz | |
| Surface electromyogram (EMG) | 1 Hz | 100 Hz | 30 s | ON | 200 Hz | |
| Chest wall respiration (THR) | 0.5 Hz | 30 Hz | 1 min | ON | 200 Hz | |
| Electrocardiogram (ECG) | 0.5 Hz | 30 Hz | 30 s | ON | 200 Hz | |
| VEP Channels | ||||||
| Cz-Oz | 12 μ/div | 1 Hz | 100 Hz | 600 ms | ON | |
| A1-Oz | 12 μ/div | 1 Hz | 100 Hz | 600 ms | ON | |
| Maximal averaging | 100 | |||||
| Stimulation | Monocular, one eye first, then the other | |||||
| Light | Red | |||||
| Intensity | Standard flash of 3 cd·s/m2 | |||||
| Stimulator type | Handheld LEDs google | |||||
| Frequency | 1 Hz | |||||
| Duration | 10 ms | |||||
Table 1: Instruments settings for recording neonatal EEG and VEP.
| Stage of the sleep-wake cycle | Predominant pattern in the EEG | Ocular movements | Surface chin electromyogram | Breathing |
| Wakefulness | Frequent artifacts by movements. Irregular low-amplitude EEG+C23 | Eyes open, blinking, transient closing when crying | Presence of large amplitudes | Irregular |
| Active sleep (AS) | Irregular low-amplitude EEG | Eyes closed with conjugated and rapid eyes movements | Absent or at minimum levels throughout recording | Irregular |
| Quiet sleep (QS) | Slow waves of large amplitude and tracé alternant | Eyes closed; absence of ocular movements | Muscle tone present is less than during wakefulness | Regular |
| Transitional sleep | 3 traits of QS and 2 of AS are present in the same 30 seconds segment or vice versa | Regular or irregular | ||
Table 2: Criteria applied for electroencephalographical detection of neonatal sleep stages.
Discussion
Three components of visual-evoked responses (NII, PII and NIII) were characterized in healthy, full-term newborns while doing stimulation with LED googles, and recorded during polygraphically-identified sleep states. The VEP morphology observed is consistent with previous results reported for fewer neonates11,15. The characterization of VEP responses was achieved by recording 20 healthy, full-term newborns at similar post-conceptional age16. This methodology allowed researchers to reduce the variability in VEP responses that has been reported previously when no control of age was applied so the study groups mixed preterm, newborns and several-months-old babies, no attention was paid to sleep states and white light strobe flash was used to elicit VEPs7,10,11,15. No significant differences were found in amplitude and latency between the VEPs obtained in QS and AS, but those recorded during the latter sleep stage are recommended because they can be obtained in 100% of newborns with fewer average repetitions required to obtain reproducibility. VEPs recorded during QS, in contrast cannot be obtained in all neonates, and require more repetitions16.
VEP morphology during AS is more reliable because the reproducibility of the characteristics of these VEPs is significantly higher, as shown by the correlation between the two VEP averages. During QS, however, morphology can differ from one average to another because atypical morphologies are often seen, and lower correlations are obtained. As a result, the risk of arriving at misleading prognoses is higher6,13.
For these reasons, recording VEPs during transitional and QS stages should be avoided. Although studying visual function in wakefulness is optimal in adults21, in newborns cephalic movements to avoid luminous stimuli, blinking and electromyographic artifacts can all compromise VEP recording. The variability introduced during QS is probably due to tracé alternant, which is present until 43-44 weeks of post-conceptional age17,18. VEP recording during AS, in contrast, is easier because the absolute power of EEG activity is lower in this sleep stage, and maximum relaxation due to muscular atonia helps avoid technical artifacts caused by movement. Finally, the use of handheld LED googles minimizes the variability introduced by eye movements and ambient light. To consult normal results of VEPs to handheld LEDs by age, see Taylor et al. and Tsuneishi and Casaer9,22.
The methodology described herein has several advantages. First, there is no need for new or sophisticated equipment, just a polygraph synchronized with luminous stimuli or simultaneously recorded polysomnography during VEP recording. Second, it is easier to identify active sleep reliably than quiet sleep or awake states; AS is easily accessible since it occurs at the beginning of spontaneous neonatal sleep and occupies 50% of the time asleep at this age17,18. Third, this approach is not time-consuming because sleep cycles in this developmental stage are very short, lasting only around 40 minutes.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Engineer Héctor Belmont, Dr. Mónica Carlier, Dr. Yuria Cruz and Dr. María Elena Juárez collaborated in data collection. The authors thank Paul Kersey for revising English language use. The project was partially funded by PAPIIT grant IN2009/7 and CONACYT (National Council for Science and Technology, Mexico) grant 4971.
Materials
| Digital Electroencephalograph | Neuronic Mexicana, SA | Medicid 3E | Sleep electroencephalogram record |
| Evoked Potentials equipment | Neuronic Mexicana, SA | Neuronic PE (N_N-SW-2.0) | Visual evoked potentials record |
| Nuprep Gel | WEAVER and Company | Skin preparing abrasive gel (114 g) | |
| Ten20 Conductive Paste | WEAVER and Company | Neurodiagnostic electrode paste (228 g) | |
| Tubular elastic mesh bandage | Le Roy | Fixation of cranial surface electrodes, Size 4 or Small | |
References
- Harmony, T., et al. Longitudinal study of children with perinatal brain damage in whom early neurohabilitation was applied: Preliminary report. Neuroscience Letter. 12 (611), 59-67 (2016).
- Spittle, A., Orton, J., Anderson, P. J., Boyd, R., Doyle, L. W. Early developmental intervention programs provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database System Review. 24 (11), CD005495 (2015).
- Huang, X., et al. Volume Changes and Correlation with Visual Evoked Potential in Patients with Optic Neuritis: A Voxel-Based Morphometry Study. Medical Science Monitor. 22, 1115-1123 (2016).
- McGlone, L., et al. Neonatal Visual Evoked Potentials in Infants Born to Mothers Prescribed Methadone. Pediatrics. 131 (3), 857-863 (2013).
- Cruz, S., Crego, A., Ribeiro, E., Goncalves, O., Sampaio, A. A VEP study in sleeping and awake one-month-old infants and its relation with social behavior. International Journal of Developmental Neuroscience. 41, 37-43 (2015).
- Kato, T., Watanabe, K. Visual evoked potential in the newborn: Does it have predictive value?. Seminars in Fetal & Neonatal Medicine. 11, 459-463 (2006).
- Shepherd, A., Saunders, K., McCulloch, D. Effect of sleep state on the flash visual evoked potential. A case study. Documenta Ophthalmologica. 98, 247-256 (2000).
- Mercuri, E., Siebenthal, K., Tutuncuoglu, S., Guzzetta, E., Casaer, P. The Effect of Behavioural States on Visual Evoked Responses in Preterm and Full-Term. Neuropediatrics. 26, 211-213 (1995).
- Taylor, M. J., Menzies, R., MacMillan, L. J., Whyte, H. E. VEPs in normal full-term and premature neonates: longitudinal versus cross-sectional data. Electroencephalography and Clinical Neurophysiology. 68, 20-27 (1987).
- Hrbek, A., Karlberg, P., Olsson, T. Development of visual and somatosensory evoked responses in pre-term newborn infants. Electroencephalography and Clinical Neurophysiology. 34, 225-232 (1973).
- Benavente, I., Tamargo, P., Tajada, N., Yuste, V., Oliva, M. J. Flash visually evoked potentials in the newborn and their maturation during the first six months of life. Documenta Ophthalmologica. 110, 255-263 (2005).
- Tsuneishi, S., Casaer, P., Fock, J. M., Hirano, S. Establishment of normal values for flash visual evoked potentials (VEPs) in preterm infants: a longitudinal study with special reference to two components of the N1 wave. Electroencephalography and Clinical Neurophysiology. 96, 291-299 (1995).
- Roy, M. S., Gosselin, J., Hanna, N., Orquin, J., Chemtob, S. Influence of the state of alertness on the pattern visual evoked potentials (PVEP) in very young infant. Brain & Development. 26, 197-202 (2004).
- Kraemer, M., Abrahamsson, M., Sjostrom, A. The neonatal development of the light flash visual evoked potential. Documenta Ophthalmologica. 99, 21-39 (1999).
- Apkarian, P., Mirmiran, M., Tijssen, R. Effects of Behavioral State on Visual Processing in Neonates. Neuropediatrics. 22, 85-91 (1991).
- Cubero-Rego, L., Corsi-Cabrera, M., Ricardo-Garcell, J., Cruz-Martínez, R., Harmony, T. Visual evoked potentials are similar in polysomnographically defined quiet and active sleep in healthy newborns. International Journal of Developmental Neuroscience. 68, 26-34 (2018).
- Mizrahi, E. M., Moshé, S. L., Hrachovy, R. A., Niedermeyer, E., Lopes da Silva, F. H. Normal EEG and Sleep: Preterm and Term Neonates. Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related. , 154-163 (2011).
- Grigg-Damberger, M. The Visual Scoring of Sleep in Infants 0 to 2 Months of Age. Journal of Clinical Sleep Medicine. 12 (3), 429-445 (2016).
- Husain, A. M., Niedermeyer, E., Lopes da Silva, F. H. Evoked Potentials in Children and Infants. In Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. , 1057-1082 (2011).
- Odom, J. V., et al. ISCEV standard for clinical visual evoked potentials: 2016 update. Documenta Ophthalmologica. 133 (1), 1-11 (2016).
- Pojda-Wilczek, D., Maruszczyk, W., Sirek, S. Flash visual evoked potentials (FVEP) in various stimulation conditions. Documenta Ophthalmologica. 138, 35-42 (2019).
- Tsuneishi, S., Casaer, P. Stepwise decrease in VEP latencies and the process of myelination in the human visual pathway. Brain & Development. 19, 547-551 (1997).

