Transcranial Direct Current Stimulation (tDCS) of Wernicke’s and Broca’s Areas in Studies of Language Learning and Word Acquisition
Summary
Here, we describe a protocol for using transcranial direct current stimulation for psycho- and neurolinguistic experiments aimed at studying, in a naturalistic yet fully controlled way, the role of cortical areas of the human brain in word learning, and a comprehensive set of behavioral procedures for assessing the outcomes.
Abstract
Language is a highly important yet poorly understood function of the human brain. While studies of brain activation patterns during language comprehension are abundant, what is often critically missing is causal evidence of brain areas' involvement in a particular linguistic function, not least due to the unique human nature of this ability and a shortage of neurophysiological tools to study causal relationships in the human brain noninvasively. Recent years have seen a rapid rise in the use of transcranial direct current stimulation (tDCS) of the human brain, an easy, inexpensive and safe noninvasive technique that can modulate the state of the stimulated brain area (putatively by shifting excitation/inhibition thresholds), enabling a study of its particular contribution to specific functions. While mostly focusing on motor control, the use of tDCS is becoming more widespread in both basic and clinical research on higher cognitive functions, language included, but the procedures for its application remain variable. Here, we describe the use of tDCS in a psycholinguistic word-learning experiment. We present the techniques and procedures for application of cathodal and anodal stimulation of core language areas of Broca and Wernicke in the left hemisphere of the human brain, describe the procedures of creating balanced sets of psycholinguistic stimuli, a controlled yet naturalistic learning regime, and a comprehensive set of techniques to assess the learning outcomes and tDCS effects. As an example of tDCS application, we show that cathodal stimulation of Wernicke's area prior to a learning session can impact word learning efficiency. This impact is both present immediately after learning and, importantly, preserved over longer time after the physical effects of stimulation wear off, suggesting that tDCS can have long-term influence on linguistic storage and representations in the human brain.
Introduction
The neurobiological mechanisms of human language function are still poorly understood. As the bedrock of our communication ability, this unique human neurocognitive trait plays a particularly important role in our personal and socio-economic lives. Any deficits affecting speech and language are devastating for the sufferers and expensive for the society. At the same time, in the clinic, procedures for treatment of speech deficits (such as aphasia) remain suboptimal, not least due to poor understanding of the neurobiological mechanisms involved1. In research, the recent advent and rapid development of neuroimaging methods have led to multiple discoveries describing activation patterns; yet, causal evidence is often still lacking. Furthermore, language areas of the brain are located somewhat suboptimally for application of mainstream neurostimulation approaches which can provide causal evidence, most importantly the transcranial magnetic stimulation technique (TMS). Whereas offline TMS protocol, such as theta burst stimulation, can cause pain due to the close proximity of the muscles to the point of stimulation, "online" TMS protocols can introduce sound artifacts from stimulation, which is undesirable due to interference with linguistic stimulus presentation2. Even though TMS is widely used in language studies despite such inconveniences, a welcome alternative may be provided by other stimulation methods, most notably transcranial direct-current stimulation (tDCS). In recent years, tDCS has seen a remarkable growth in its use due to its accessibility, ease of use, relative safety and often rather striking outcomes3. Even though the exact mechanisms underpinning tDCS influence on neural activity are not understood completely, the mainstream view is that, at least at low intensity levels (typically 1-2 mA for 15-60 min), it does not cause any neural excitation or inhibition per se, but instead modulates the resting transmembrane potential in a graded way towards de- or hyperpolarization, shifting the excitation thresholds up or down and thereby making the neural system more or less susceptible to modulations by other events, stimuli, states or behaviors4,5. Whereas most of the applications reported to date have focused on the motor function6 and/or motor system deficits, it has been increasingly applied to higher-level cognitive functions and their respective disabilities. There has been a rise in its application to speech and language, mostly in research aimed at the recovery of post-stroke aphasia7,8,9, even though it has so far led to mixed results with respect to the therapeutic potential, stimulation sites and hemispheres, and optimal current polarity. As this research, and particularly the application of tDCS in cognitive neurobiology of normal language function, is still in its infancy, it is crucial to delineate procedures for stimulating at least the core language cortices (most importantly Wernicke's and Broca's areas) using tDCS, which is one of the main aims of the current report.
Here, we will consider application of tDCS to language areas in a word-learning experiment. In general, the case of word learning is taken here as one example of a neurolinguistic experiment, and the tDCS part of the procedure should not change substantially for other types of language experiments targeting the same areas. Yet, we use this opportunity to also highlight major methodological considerations in a word acquisition experiment per se, which is the second main aim of the current protocol description. Brain mechanisms underpinning word acquisition – a ubiquitous human capacity at the core of our linguistic communication skill – remain largely unknown10. Complicating the picture, existing literature differs widely in how experimental protocols promote word acquisition, in control over stimulation parameters, and in tasks used to assess learning outcomes (see, e.g., Davis et al.11). Below, we describe a protocol that uses highly controlled stimuli and presentation mode, while ensuring a naturalistic context-driven acquisition of novel vocabulary. Furthermore, we use a comprehensive battery of tasks to assess the outcomes behaviorally at different levels, both immediately after learning and following an overnight consolidation stage. This is combined with sham and cathodal tDCS of language areas (we make a particular example using Wernicke's area stimulation) which can provide causal evidence on underlying neural processes and mechanisms.
Protocol
All procedures were approved by the local research ethics committee of St. Petersburg State University, St. Petersburg, with consent obtained from all participants.
NOTE: All participants must sign the informed consent and fill in a questionnaire to attest the absence of any contraindications for tDCS stimulation (see Technique and Considerations in the Use of 4 x 1 Ring High-definition Transcranial Direct Current Stimulation (HD-tDCS) by Willamar and colleagues12) and to collect other data relevant to the study such as vision acuity, demographics, language experience and handedness. For the latter, the seminal work by Oldfield13 is recommended.
1. Subjects and experimental environment
- In a typical language experiment, ensure that all subjects are right-handed and have no record of language deficits, neurological or psychiatric disorders. Their native language and bilingual/multilingual status must be controlled.
- Conduct all measurements in a sound-proof or at least sound-attenuated chamber. Sound insulation is very important, since any extraneous sound, noise, human speech, etc. can significantly affect the performance and thus influence the data (Figure 1).
- To avoid interference by unnecessary subject-experimenter contact, place only the screen, headphones/speakers and any input devices (keyboard, button boxes) inside the chamber. Have all interaction with the experimenter over intercom unless personal contact is required.
- Use the following optimal parameters, based on extensive piloting, for background color and font size: grey background color (RGB: 125, 125, 125), black text color (RGB: 0; 0; 0), Arial font face, size 27.
- To reduce delays and jitter in visual presentation, use a video card and a monitor with a refresh rate of 100 Hz and higher.
- To measure reaction times, use research-grade response pads, which have better ergonomics and more precise timing in comparison with conventional keyboards.
2. Stimulus preparation
- Choose words of the language in question, which are controlled for their duration, lexical frequency and overall structure (to avoid any basic effects of surface stimulus properties on higher-level processing). Here, all base words were eight phonemes/letters long and consisted of three syllables with the CVCCVCVC structure (where C is consonant, and V is vowel).
- To create multiple lists, divide the words into sets, which should not differ statistically (as measured with, e.g., t-tests) on their lemma, bigram and/or trigram as well as syllabic frequency. These can be obtained from language-specific psycholinguistic databases; here, Russian National Corpus was used (http://www.ruscorpora.ru/en/). Here, one set was used for creating (through modification) orthographically similar novel words and pseudowords, another set for creating unrelated control pseudowords, and a further set used as unrelated control words (Figure 2A). This led to five sets of 10 items each (50 stimuli in total). Modify these procedures in accordance with your exact experimental requirements.
- To minimize any effects of surface forms on newly acquired semantics, counterbalance the sets across the subject sample, such that they play different experimental roles for different subjects.
- Create novel word forms such that they follow the rules of phonology and phonotactics and resemble existing words in terms of orthographic and phonological structure.
NOTE: To make sure that the new words can enter into competition with existing words, the current procedures were based on those developed in a series of experiments by Gaskell and colleagues11,14 and aimed at keeping the word onsets (CVCCV-) stable, while rotating their offsets (-CVC) across different items in the set. That is, we preserved the first two syllables of an existing word and varied the ultimate syllable such that a new, previously unfamiliar novel word form was created (e.g., mandarin -> mandanal*, where the last CVC was taken from another word in the list, cardinal, to create a new item). - Repeat the procedure described above for creating as many novel word forms as needed. For the current demonstration, we created lists of novel word forms to be learnt and of similar unlearnt pseudowords (e.g. mandarin -> mandanal*, mandaket*, all three potentially entering into a lexical competition post-learning, as neighbors) as well as further control lists of real words and novel pseudowords that did not share this similarity and thus would not produce a lexical competition with the main stimuli (e.g., circular, muskenal*; Russian examples are used throughout, transliterated from Cyrillic to Latin script for ease of understanding).
3. Sentence stimuli for contextual semantic learning
- Create novel meanings to be associated with the new words in the process of learning. This could be made-up, obsolete or rare objects or concepts not present in the subjects' native language or culture.
- For contextual learning of novel semantics, the procedures used by Mestrez-Misse and colleagues15 are recommended. Create several unique sentences that describe situations through which one can understand the meaning of each of the novel words (e.g., "To control insects in medieval times, people used mandaket"). Use a sequence of such sentences for each of the novel words (here, a total of 5 sentences per word), and gradually reveal the meaning of each new concept from a more general to more specific sentential context.
- Present novel words ideally in their dictionary form (i.e., uninflected, e.g., singular nominative or accusative case in Russian), such that the surface form is not inflected differently in different sentences (Table 1), unless inflection rule learning is also required.
- Control and balance the length of the sentences and the number of words between conditions. Here, each sentence consisted of 8 words. Always place novel words at the end of the sentences. Such placement allows build-up of necessary contextual information (further, this allows implementing this design, if needed, in an EEG or MEG setting to record evoked brain responses unmasked by further word stimuli).
- Present word-specific sentences in word-specific sub-blocks, gradually revealing the meaning of each new word, without interleaving or randomizing sentences related to different novel words.
- Randomize the order of the sub-blocks across the subject group. Word-by-word sentence presentation is recommended if the visual modality is used.
- Determine the interstimulus interval based on specific stimulus properties to allow their convenient presentation (Figure 2B); make sure to separate different sub-blocks with additional intervals and give regular breaks.
4. Tasks to assess acquisition of new word forms and novel meanings
NOTE: Use several tasks to assess different levels of acquisition and comprehension of both surface word forms and lexical semantics. Five tasks are used in the present protocol: free recall, cued recognition, lexical decision, semantic definition and semantic matching. The tasks are applied in the order they are listed below, which was optimized to reduce any carryover between successive tasks.
- In the free recall task, have each participant reproduce as many new word forms as they could remember by typing them into the prepared spreadsheet. The instruction is as follows: "Please write down in the column all the new words that you can remember."
- Include the same stimuli in the recognition and lexical decision (second and third tasks, respectively) and use the same presentation rate.
- These tasks include all items (novel words, real competitor words the novel ones are derived from, untrained pseudoword competitors derived from the same real words, unrelated control pseudowords and unrelated control existing words).
- For the recognition task, use the following instruction: "You will be presented with words sequentially. Press button "1" with the middle finger of the left hand if you have encountered the word during the experiment, or press "2" with the index finger of the left hand if you have not." Modify the response coding, hand and fingers in accordance with your specific requirements.
- The instruction for the lexical decision task is: "You will be presented with real and meaningless words sequentially. Press "1" with the middle finger of the left hand if the word makes sense, or press "2" with the index finger of the left hand if it does not." Modify these as necessary.
- Use the semantic definition task to estimate the acquisition of novel meaning and the correspondence between the meaning and the surface form.
- Give participants a list of the learnt items (i.e., those presented previously in the learning phase) with the instruction above: "Here is a list of new words presented to you previously. Try to define each of them and type their definitions into the spreadsheet".
- To assess the completeness and accuracy of the given definitions, engage independent experts to rate the responses; agreement between experts could be tested using, e.g., Kendall's coefficient of concordance (W).
- Use semantic matching task to assess the acquisition of semantics through making explicit links between the newly learnt word forms and their meanings in a simplified manner.
- Use the following instruction: "You will be presented a word and three definitions. You should choose one correct definition for each word by pressing the corresponding button". Only one of the definitions is correct, with the other two corresponding to the other novel items. In addition to the three optional definitions, including "none of this" or/and "not sure" options is also recommended.
5. Procedures
- Ensure that the tDCS stimulation precedes the behavioral task it is intended to modulate.
- Wernicke's area.
NOTE: The stimulation electrode placement that best corresponds to Wernicke's area is CP5 according to the extended International 10-20 system for EEG16,17.- To locate this location in the absence of an electrode cap, follow the standard 10-20 system procedures.
- Measure the head with a tape from the inion to the nasion, and note the middle of this distance. Then, measure the distance from the left preauricular point to the right preauricular point, and mark the crosspoint of the two measurements.
- To find the CP5 location, measure 30% of the distance between the preauricular points from the crosspoint down the left hemisphere and mark it. Measure 10% of the distance between the inion and the nasion from the marked point to the back of the head. This point is the CP5 location for the active electrode (Figure 3).
- Broca's area
NOTE: Closest to Broca's area is the F5 electrode site18 according to the 10-20 system.- In the absence of an EEG cap, follow the standard 10-20 system procedures to find and mark the crosspoint between inion-nasion and preauricular points, as described above.
- To find the F5 location, measure 20% of the distance between the inion and the nasion from the crosspoint to the front of the head. Measure 30% of the distance between the preauricular points from the recently marked point down the left hemisphere. This point corresponds to the F5 location for the active electrode (Figure 3).
- Homologous locations in the right hemisphere: for right-hemispheric homologues of Wernicke's and Broca's areas, use the same procedures as above, with the exception of measuring the distance from the midline down the right side of the scalp. Electrode locations are: CP6 for the RH Wernicke homologue and F6 for the Broca homologue.
- Use spongy electrodes measuring 5 cm x 5 cm as this size is a good compromise between focal stimulation (which causes more irritation and discomfort) and larger electrodes that lack focality. Soak the electrodes in physiological saline solution for 5 min before application.
- In order to minimize the effect of stimulation on other areas of the brain, place the reference electrode at the base of the neck on the left (right for homologues) side (see Figure 3 and Figure 4). Use spongy electrodes measuring 5 cm x 5 cm as well.
NOTE: Particular attention should be paid to preventing the spreading of the solution beyond the boundaries of the electrode application zone. Special care should be taken to keep the surrounding electrode area dry. - For optimal cathodal stimulation, use 1.5 mA current for 15 min. At the onset, the current gradually rises from 0 to 1.5 mA over 30 s, and at the end of the stimulation it drops back to zero over 30 s.
- For anodal stimulation, use the same procedure as cathodal stimulation, except the polarity is reversed, and the anodal electrode is placed at the active site, while the cathode is used as the reference electrode located outside the scalp area.
- Wernicke's area.
- Sham stimulation
- Perform the sham stimulation procedure generally as described above except that the current is only applied briefly in the beginning and the end of the sham session. To this end, during the first and the last 30 s of the session, apply an electric pulse of a triangular shape with a maximum of 1.5 mA, as used in the present protocol.
- Main behavioral task: contextual semantic learning
- Present sets with contextual sentences for the novel words in a random order. Start each sentence with a word-by-word presentation.
- After this, display the entire sentence on the screen to ensure its full understanding. Have participants press the spacebar with the index finger of the left hand after reading the whole sentence. Duration of sentence presentation is 5000 ms.
NOTE: The sets of the sentences are separated from each other by appearance of three crosshairs ("+++") for 2000 ms. Each new concept presentation starts with a single fixation cross ("+") present for 500 ms before the sentence words are flashed. Each word is presented for 500 ms, and the empty screen in the background color between words within one sentence is 300 ms long.
- Acquisition assessment procedure
- To assess learning effects both immediately and following the overnight consolidation stage, break the stimulus set into two subsets, equally distributed across stimulus conditions and counterbalanced across the subject group, and run the assessment task immediately after the learning protocol on one subset, and after a 24 h delay on the other one.
NOTE: This strategy is based on the literature that highlights the importance of overnight memory consolidation for the acquisition of new words19,20. - Use all developed tasks in the order described in section 3 above to assess different levels of word/concept acquisition. Choose the order of the tasks to minimize any carryover effects from one task to the following ones.
- For Tasks 1 and 4 use spreadsheets to be filled by subjects (by hand or using a text or spreadsheet processor); present the other tasks using temporally precise simulation software.
NOTE: Each stimulus in Tasks 2 and 3 is presented for 600 ms, with a fixation cross ("+") present in the interstimulus interval (1400 ms); see Figure 3. For the other tasks the response time is not limited.
- To assess learning effects both immediately and following the overnight consolidation stage, break the stimulus set into two subsets, equally distributed across stimulus conditions and counterbalanced across the subject group, and run the assessment task immediately after the learning protocol on one subset, and after a 24 h delay on the other one.
6. Data analysis
- Perform data analysis using different tests comparing two sets of samples coming from continuous distributions (such as Wilcoxon signed rank test or Mann-Whitney U-test) or medians (two-sample t-test, if the distribution is normal).
Representative Results
While the data were analyzed for the specific set of tasks, it should be emphasized that the developed set of tests and the paradigm could be adapted to a variety of psycholinguistic experiments. The results were analyzed in terms of accuracy scores (number of correct answers) and the reaction time (RT) using non-parametric Wilcoxon signed rank test and Mann-Whitney U test across groups (cathodal and sham stimulation conditions). Significant differences for tasks within each group are presented in Table 3; below, we highlight the main stimulation-related results (for descriptive statistic see Table 2).
The comparison of performance in lexical decision task between the two groups (cathodal versus sham stimulation conditions) showed differences on the first day between accuracy for competitor pseudowords: accuracy increased more after cathodal than after sham stimulation (р ≤ 0.041), suggesting reduced lexical competition after cathodal stimulation. In the recognition task, accuracy for novel words was better after sham than after cathodal stimulation both on the first (р ≤ 0.034) and on the second (р ≤ 0.09) day, suggesting reduced lexical learning efficiency after stimulation. Neither of the tasks showed differences in RT between groups. The results of the semantic tasks showed the matching between the novel form meaning and the surface form was more successful for cathodal group over sham on the second day only (р ≤ 0.011).
Within each group, there were notable differences in accuracy scores and reaction times between the two assessment sessions. In the sham group, novel word recognition was better on the first than on the second day (р ≤ 0.049). In the cathodal group, RT in the recognition task was significantly shorter for novel words than for competitor pseudowords on the first day (р ≤ 0.042), but not on the second one. The results of lexical decision task showed that after cathodal stimulation on the first (р ≤ 0.003) and on the second day (р ≤ 0.001), there was better performance for novel words than for pseudoword competitors. In the sham group, however, this effect was observed on the second day only (р ≤ 0.002).

Figure 1: Experimental chamber. Please click here to view a larger version of this figure.
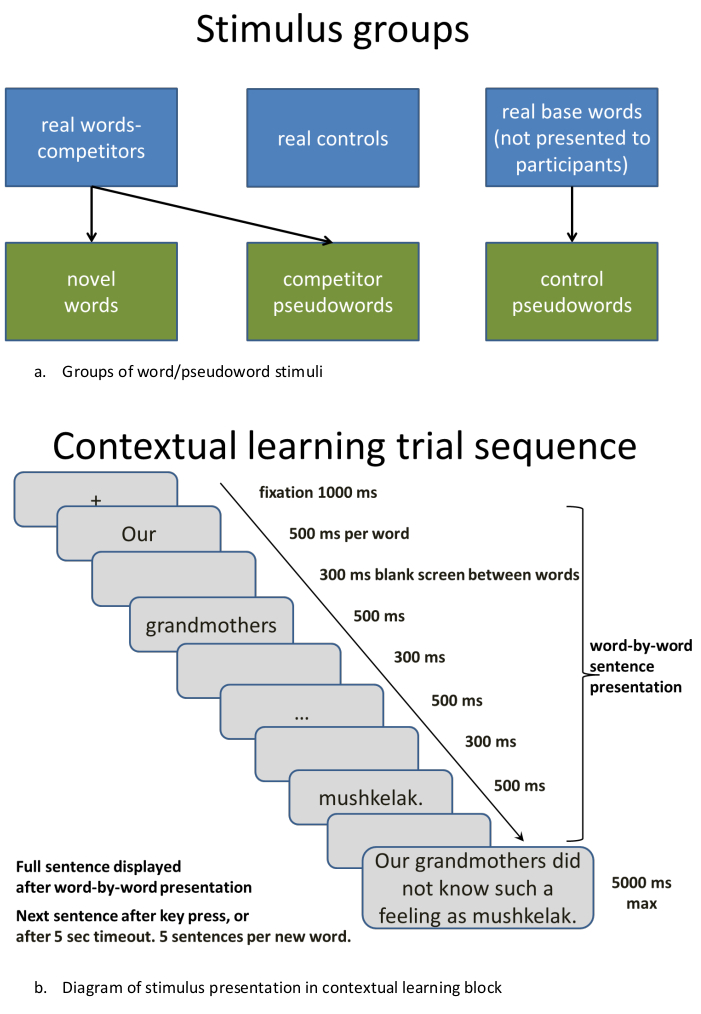
Figure 2: Procedure for presenting stimuli in contextual learning sequence. (A) Making stimulus groups: Groups of word/pseudoword stimuli. (B) Diagram of stimulus presentation in contextual learning block. Please click here to view a larger version of this figure.
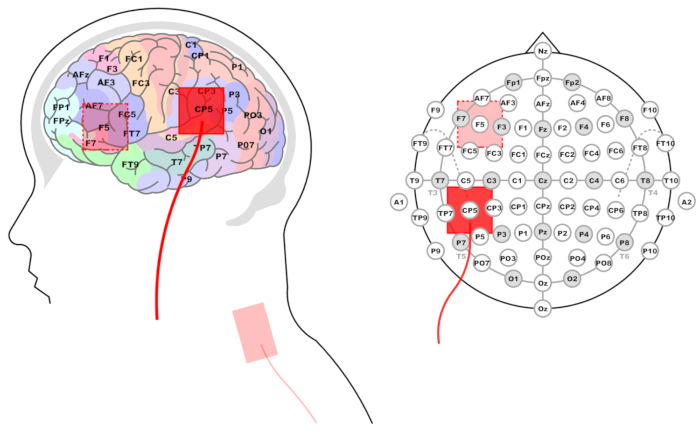
Figure 3: Location of stimulation electrode for the Wernicke's and Broca's areas. Left panel: Side view and projection on brain areas. Brain zones, EEG electrodes (system 10-20%) corresponding to them, and red rectangles representing the location of stimulating electrodes are marked. The reference electrode is shown at the base of the neck. Right panel: Projection of the stimulating electrode on the EEG 10-20% system layout. Please click here to view a larger version of this figure.

Figure 4: tDCS equipment. (A) Stimulator; (B) saline; (C) electrodes Please click here to view a larger version of this figure.
| Examples of sentences |
| Нашим бабушкам было неведомо такое чувство как мушкелак. Our grandmothers did not know such a feeling as mushkelak. |
| Благодаря своей хорошей памяти, Маша не чувствовала мушкелак. Thanks to her good memory, Masha never experienced any mushkelak. |
| Заведя сразу несколько аккаунтов, я начал испытывать мушкелак. Having got a few accounts, I started suffering from mushkelak. |
| Секретный блокнот поможет решить такую проблему как мушкелак. A secret notebook could help you solve the problem of mushkelak. |
| Петр устанавливал одинаковые пароли, не желая ощущать мушкелак. Peter always set the same password as he did not want to have any mushkelak. |
Table 1: Examples of sentences for contextual learning of novel words.
| Sham stimulation | Cathodal stimulation | ||||
| Mean | SD | Mean | SD | ||
| Task 1: free recall | |||||
| Day 1 | Accuracy | 4.91 | 2.22 | 5.69 | 1.49 |
| Day 2 | Accuracy | 2.53 | 2.44 | 2.84 | 2.26 |
| Task 2: recognition. Accuracy scores | |||||
| Day 1 | Novel words | 3.06 | 0.89 | 1.96 | 1.68 |
| Competitor words | 3.63 | 1.14 | 3.73 | 1.29 | |
| Competitor pseudowords | 2.60 | 1.15 | 2.69 | 1.39 | |
| Control pseudowords | 3.79 | 1.32 | 3.92 | 1.41 | |
| Control words | 4.67 | 1.05 | 4.29 | 1.16 | |
| Day 2 | Novel words | 2.58 | 0.93 | 1.56 | 1.47 |
| Competitor words | 4.40 | 0.74 | 4.10 | 1.39 | |
| Competitor pseudowords | 3.13 | 1.25 | 3.31 | 1.00 | |
| Control pseudowords | 4.33 | 0.92 | 4.50 | 1.14 | |
| Control words | 4.58 | 1.02 | 4.38 | 1.44 | |
| Task 2: recognition. Reaction time (ms) | |||||
| Day 1 | Novel words | 793 | 167 | 858 | 183 |
| Competitor words | 804 | 151 | 845 | 179 | |
| Competitor pseudowords | 883 | 261 | 962 | 306 | |
| Control pseudowords | 849 | 201 | 833 | 234 | |
| Control words | 699 | 131 | 767 | 196 | |
| Day 2 | Novel words | 836 | 200 | 933 | 272 |
| Competitor words | 816 | 239 | 818 | 213 | |
| Competitor pseudowords | 859 | 281 | 924 | 236 | |
| Control pseudowords | 818 | 280 | 866 | 265 | |
| Control words | 734 | 212 | 817 | 234 | |
| Task 3: lexical decision. Accuracy scores | |||||
| Day 1 | Novel words | 2.42 | 1.63 | 1.96 | 1.68 |
| Competitor words | 4.13 | 0.78 | 4.10 | 0.90 | |
| Competitor pseudowords | 3.46 | 1.17 | 4.02 | 1.33 | |
| Control pseudowords | 4.21 | 1.02 | 4.25 | 1.26 | |
| Control words | 4.54 | 0.72 | 4.54 | 0.78 | |
| Day 2 | Novel words | 2.04 | 1.47 | 1.56 | 1.47 |
| Competitor words | 4.38 | 0.56 | 4.46 | 0.61 | |
| Competitor pseudowords | 3.81 | 1.08 | 3.94 | 1.39 | |
| Control pseudowords | 4.54 | 0.78 | 4.58 | 1.28 | |
| Control words | 4.42 | 0.72 | 4.63 | 0.71 | |
| Task 3: lexical decision. Reaction time (ms) | |||||
| Day 1 | Novel words | 817 | 244 | 921 | 248 |
| Competitor words | 747 | 181 | 797 | 201 | |
| Competitor pseudowords | 927 | 307 | 910 | 265 | |
| Control pseudowords | 891 | 291 | 852 | 213 | |
| Control words | 737 | 217 | 784 | 221 | |
| Day 2 | Novel words | 878 | 287 | 963 | 292 |
| Competitor words | 743 | 174 | 811 | 197 | |
| Competitor pseudowords | 914 | 290 | 918 | 244 | |
| Control pseudowords | 871 | 286 | 853 | 244 | |
| Control words | 719 | 189 | 756 | 234 | |
| Task 4: semantic definition | |||||
| Day 1 | Matching | 1.27 | 0.75 | 1.87 | 1.45 |
| Accuracy | 7.97 | 4.03 | 8.71 | 5.66 | |
| Day 2 | Matching | 0.52 | 0.79 | 1.39 | 1.44 |
| Accuracy | 2.82 | 2.73 | 5.86 | 5.74 | |
| Task 5: semantic matching | |||||
| Day 1 | Accuracy | 3.16 | 0.97 | 3.18 | 1.03 |
| Reaction time (ms) | 10914 | 3391 | 10856 | 6039 | |
| Day 2 | Accuracy | 2.41 | 1.07 | 2.89 | 1.25 |
| Reaction time (ms) | 8798 | 2488 | 8908 | 3419 | |
Table 2: Descriptive statistics.
| Sham stimulation | p-value | Cathodal stimulation | p-value | |
| Task 1: free recall. Accuracy scores | ||||
| Between days | Accuracy scores Day 1 vs. accuracy scores Day 2 | 0.001 | Accuracy scores Day 1 vs. accuracy scores Day 2 | <0.001 |
| Task 2: recognition. Accuracy scores | ||||
| Day 1 | Novel words vs. | ─ | Novel words vs. | ─ |
| Competitor words | 0.042 | Competitor words | 0.004 | |
| Control pseudowords | 0.041 | Competitor pseudowords | 0.045 | |
| Control words | 0.001 | Control pseudowords | 0.002 | |
| ─ | ─ | Control words | <0.001 | |
| Day 2 | Novel words vs. | ─ | Novel words vs. | ─ |
| Competitor words | 0.001 | Competitor words | <0.001 | |
| Control pseudowords | 0.001 | Competitor pseudowords | 0.001 | |
| Control words | 0.001 | Control pseudowords | <0.001 | |
| ─ | ─ | Control words | <0.001 | |
| Between days | Novel words | 0.049 | Competitor words | 0.036 |
| Competitor words | 0.011 | Competitor pseudowords | 0.024 | |
| Competitor pseudowords | 0.034 | Control pseudowords | 0.020 | |
| Control pseudowords | 0.030 | ─ | ─ | |
| Recognition. Reaction time (ms) | ||||
| Day 1 | Novel words vs. Control words | 0.005 | Novel words vs. | ─ |
| Competitor pseudowords | 0.042 | |||
| Control words | 0.006 | |||
| Day 2 | Novel words vs. Control words | 0.007 | Novel words vs. | ─ |
| Competitor words | 0.001 | |||
| Control pseudowords | 0.045 | |||
| Control words | 0.014 | |||
| Task 3: lexical decision. Accuracy scores | ||||
| Day 1 | Novel words vs. | ─ | Novel words vs. | ─ |
| Competitor words | 0.001 | Competitor words | <0.001 | |
| Control pseudowords | 0.001 | Competitor pseudowords | 0.003 | |
| Control words | 0.001 | Control pseudowords | 0.001 | |
| ─ | ─ | Control words | <0.001 | |
| Day 2 | Novel words vs. | ─ | Novel words vs. | ─ |
| Competitor words | 0.001 | Competitor words | <0.001 | |
| Competitor pseudowords | 0.002 | Competitor pseudowords | 0.001 | |
| Control pseudowords | 0.001 | Control pseudowords | <0.001 | |
| Control words | 0.001 | Control words | <0.001 | |
| Between days | No significant differences | ─ | Control pseudowords | 0.033 |
| Lexical decision. Reaction time (ms) | ||||
| Day 1 | Novel words vs. | Novel words vs. | ─ | |
| Competitor words | 0.022 | Competitor words | 0.001 | |
| Competitor pseudowords | <0.001 | Control words | 0.013 | |
| Control pseudowords | 0.033 | ─ | ─ | |
| Day 2 | Novel words vs. | ─ | Novel words vs. | ─ |
| Competitor words | 0.003 | Competitor words | 0.003 | |
| Control words | 0.008 | Control words | 0.001 | |
| Task 4: semantic definition. Matching and Accuracy scores | ||||
| Between days | Matching scores Day 1 vs. matching scores Day 2 | 0.001 | Matching scores Day 1 vs. matching scores Day 2 | 0.006 |
| Accuracy scores Day 1 vs. accuracy scores Day 2 | 0.001 | Accuracy scores Day 1 vs. accuracy scores Day 2 | <0.001 | |
| Task 5: semantic matching. Accuracy scores | ||||
| Between days | Accuracy scores Day 1 vs. accuracy scores Day 2 | 0.006 | No significant differences | ─ |
| Semantic matching. Reaction time (ms) | ||||
| Between days | Reaction time Day 1 vs. reaction time Day 2 | 0.002 | Reaction time Day 1 vs. reaction time Day 2 | 0.015 |
Table 3: Significant differences in accuracy scores and reaction times within each group (sham and cathodal stimulations). The values in parentheses are the mean scores and the reaction times.
Discussion
The results highlight a few important points that need to be taken into account when conducting psycholinguistic research in general, and neurolinguistics tDCS studies in particular. Stimulation of language cortices (exemplified here by Wernicke's area) produces a complex pattern of behavioral outcomes. Unlike the TMS technique, where it is possible to fully disrupt speech processing (e.g., the so-called "speech arrest" protocol)21, this method enables a possibly more complex, graded and subtle influence on the language processing mechanisms. We have found a variety of both accuracy and reaction time differences which diverged substantially between conditions, tests and assessment days. The technical implications of the protocol reported are briefly discussed below.
To disengage the various effects, a battery of different tests is needed, which could test for processes at different levels of short- and long-term memory, lexical access, semantic processing, etc. For example, the effects here include different performance in recall and recognition for different stimulus types and stimulation conditions, which suggests differential lexical competition effects for novel and old items, and diverging effects of tDCS at lexical and semantic levels. Our results confirm sensitivity of the utilized tasks to efficiency of novel word acquisition at different levels, including recognition, understanding of a word meaning and free recall.
In the same way, a tDCS condition (e.g., anodal, cathodal stimulation) requires a proper control condition (or control group), sham (placebo) stimulation being the most appropriate baseline. Unlike electrical stimulation of the motor cortex, the effects may not always be unambiguous22, they strongly depend on the tests used, or may not appear at all23.
Another very important point is that only one type of stimulation could be applied in each individual subject in the context of a single experimental session. This normally entails a between-group design, for instance, an anodal stimulation group, a cathodal stimulation group, and a placebo (sham) control group. For within-group designs, use different tDCS protocol on different days, at least 24 h apart (in learning studies, this also entails using different linguistic stimuli on different days to avoid contamination of results by repetition effects). The present report uses an experiment with cathodal stimulation of Wernicke's areas as an example, but similar procedures apply to other polarities/sites.
Contextual presentation of new words significantly expands the possibilities of simultaneous study of acquisition of word form per se and of its semantics. Traditionally, these processes are studied separately focusing either on the acquisition of a new word form or on the correlation of a meaning of a familiar word with other semantic units24,25,26. The proposed protocol combines both aims; therefore, it is possible to compare the dynamics of a new concept acquisition at the level of word form perception and that of mastering its content, which is achieved by using a comprehensive set of tests. The need for such a comparison is emphasized here by diverging dynamics of performance on novel surface forms recall and recognition as opposed to semantic matching.
It is important to remember the main differences between tDCS and other non-invasive brain stimulation methods, such as TMS. Since there is no simple way to determine individual sensitivity to tDCS by threshold assessment, a single protocol is applied for all subjects. It is very difficult to accurately estimate the stimulation area – one can only speak about the approximate/hypothetical area being stimulated. It is also difficult to estimate the duration of offline stimulation effects after the current is turned off. Presumably, the main effects of stimulation are observed up to one hour after the termination of stimulation. However, the effects can sometimes be detected even one day after the stimulation20.
Yet, compared with TMS, the relative ease of application of tDCS, the substantially lower risk of side effects and the absence of acoustic artifacts make this protocol attractive for studying the speech and language function. It is also worth noting that the combination of electrical stimulation with other methods, for example with TMS, fMRI, EEG or pharmacological intervention, allows studying neuronal mechanisms of tDCS in more detail27,28.
Since tDCS stimulation is not highly localized, a non-specific effect is possible. This is obvious from the existing evidence, where very different or even opposite protocols may sometimes lead to similar results. This may be due to the general impact on other cognitive functions and processes such as attention, retrieval from memory, and so on. A specialized battery of tests is needed to detect the effects associated with a particular language feature. Following the proposed steps of the stimulus material creation (verification of the surface or lemma frequency of the words, length of words and sentences, etc.), it is necessary to consider the grammatical and phonetic structure of a language. For instance, the number of words in a sentence and the length of the words can vary depending on the exact need. In addition, the words used in the experiment should be controlled for both spelling and sound. In an orthographically transparent language such as Russian, this is relatively straightforward, but it may be difficult to attain in other languages (e.g., English, Danish or Mandarin).
In line with a body of previous studies, we find different effects of acquisition immediately after the learning block and after an overnight sleep, which highlights effects of overnight consolidation. Importantly, we also find group differences (sham versus cathode) on the second day. It is generally accepted that the physical effect of stimulation of the cortex is relatively short-lasting, on the order of minutes to several hours. This implies that the cognitive effects achieved during the transient stimulation phase are nevertheless maintained over a longer period and may therefore be possibly used for modulating word acquisition and processing in practical settings. Obviously, not only the core language areas of Broca and Wernicke are involved in the language function; adoption of the protocol described above is possible for any area of the brain, while a battery of psycholinguistic tests fine-tuned for specific experimental purposes is still needed to assess the stimulation impact on a specific neurolinguistic trait.
Divulgations
The authors have nothing to disclose.
Acknowledgements
Supported by RF Government grant contract No.14.W03.31.0010. We wish to thank Ekatarina Perikova and Alexander Kirsanov for their support in preparing this publication. We are grateful to Olga Shcherbakova and Margarita Filippova for their help in stimulus selection and to Anastasia Safronova and Pavel Inozemcev for their assistance in the production of video materials.
References
- Sebastian, R., Tsapkini, K., Tippett, D. C. Transcranial direct current stimulation in post stroke aphasia and primary progressive aphasia: Current knowledge and future clinical applications. Neuro Rehabilitation. 39 (1), 141-152 (2016).
- Antal, A., et al. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clinical Neurophysiology. 128 (9), 1774-1809 (2017).
- Lefaucheur, J. P., et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology. 128 (1), 56-92 (2017).
- Priori, A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clinical Neurophysiology. 114 (4), 589-595 (2003).
- Shah, P. P., Szaflarski, J. P., Allendorfer, J., Hamilton, R. H. Induction of neuroplasticity and recovery in post-stroke aphasia by non-invasive brain stimulation. Frontiers in Human Neuroscience. 7, 888 (2013).
- Nitsche, M. A., et al. Modulation of cortical excitability by weak direct current stimulation–technical, safety and functional aspects. Supplements to Clinical Neurophysiology. 56, 255-276 (2003).
- Fridriksson, J., Richardson, J. D., Baker, J. M., Rorden, C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 42 (3), 819-821 (2011).
- Flöel, A., et al. Short-term anomia training and electrical brain stimulation. Stroke. 42 (7), 2065-2067 (2011).
- Hamilton, R. H., Chrysikou, E. G., Coslett, B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain and Language. 118 (1-2), 40-50 (2011).
- Shtyrov, Y. Neural bases of rapid word learning. The Neuroscientist. 18 (4), (2012).
- Davis, M. H., Di Betta, A. M., Macdonald, M. J. E., Gaskell, M. G. Learning and Consolidation of Novel Spoken Words. Journal of Cognitive Neuroscience. 21 (4), 803-820 (2009).
- Villamar, M. F., et al. Technique and Considerations in the Use of 4×1 Ring High-definition Transcranial Direct Current Stimulation (HD-tDCS). Journal of Visualized Experiments. (77), (2013).
- Oldfield, R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9 (1), 97-113 (1971).
- Rodd, J. M., et al. Learning new meanings for old words: effects of semantic relatedness. Memory & Cognition. 40 (7), 1095-1108 (2012).
- Quiroga, R. Q., Fried, I., Koch, C. Brain cells for grandmother. Scientific American. 308 (2), 30-35 (2013).
- Mason, R. A., Prat, C. S., Just, M. A. Neurocognitive brain response to transient impairment of Wernicke’s area. Cerebral Cortex (New York, N.Y.: 1991). 24 (6), 1474-1484 (2014).
- Chatrian, G. E., Lettich, E., Nelson, P. L. Modified nomenclature for the “10%” electrode system. Journal of Clinical Neurophysiology. 5 (2), 183-186 (1988).
- Nishitani, N., Schürmann, M., Amunts, K., Hari, R. Broca’s Region: From Action to Language. Physiology. 20 (1), 60-69 (2005).
- Dumay, N., Gareth Gaskell, M. Overnight lexical consolidation revealed by speech segmentation. Cognition. 123 (1), 119-132 (2012).
- Landi, N., et al. Neural representations for newly learned words are modulated by overnight consolidation, reading skill, and age. Neuropsychologia. 111, 133-144 (2018).
- Tarapore, P. E., et al. Language mapping with navigated repetitive TMS: Proof of technique and validation. NeuroImage. 82, 260-272 (2013).
- Jacobson, L., Koslowsky, M., Lavidor, M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research. 216 (1), 1-10 (2012).
- Malyutina, S., et al. Modulating the interhemispheric balance in healthy participants with transcranial direct current stimulation: No significant effects on word or sentence processing. Brain and Language. 186, 60-66 (2018).
- Geranmayeh, F., Leech, R., Wise, R. J. S. Semantic retrieval during overt picture description: Left anterior temporal or the parietal lobe?. Neuropsychologia. 76, 125-135 (2015).
- Lambon Ralph, M. A., Pobric, G., Jefferies, E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cerebral Cortex (New York, N.Y.: 1991). 19 (4), 832-838 (2009).
- Mueller, S. T., Seymour, T. L., Kieras, D. E., Meyer, D. E. Theoretical Implications of Articulatory Duration, Phonological Similarity, and Phonological Complexity in Verbal Working Memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 29 (6), 1353-1380 (2003).
- Bachtiar, V., Near, J., Johansen-Berg, H., Stagg, C. J. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. eLife. 4, e08789 (2015).
- Márquez-Ruiz, J., et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proceedings of the National Academy of Sciences of the United States of America. 109 (17), 6710-6715 (2012).

