Gene Transfer into the Chicken Auditory Organ by In Ovo Micro-electroporation
Summary
The auditory organ mediates hearing. Here we present a modified in ovo micro-electroporation method optimized for studying auditory progenitor cell proliferation and differentiation in the developing chicken auditory organ.
Abstract
Chicken embryos are ideal model systems for studying embryonic development as manipulations of gene function can be conducted with relative ease in ovo. The inner ear auditory sensory organ is critical for our ability to hear. It houses a highly specialized sensory epithelium that consists of mechano-transducing hair cells (HCs) and surrounding glial-like supporting cells (SCs). Despite structural differences in the auditory organs, molecular mechanisms regulating the development of the auditory organ are evolutionarily conserved between mammals and aves. In ovo electroporation is largely limited to early stages at E1 – E3. Due to the relative late development of the auditory organ at E5, manipulations of the auditory organ by in ovo electroporation past E3 are difficult due to the advanced development of the chicken embryo at later stages. The method presented here is a transient gene transfer method for targeting genes of interest at stage E4 – E4.5 in the developing chicken auditory sensory organ via in ovo micro-electroporation. This method is applicable for gain- and loss-of-functions with conventional plasmid DNA-based expression vectors and can be combined with in ovo cell proliferation assay by adding EdU (5-ethynyl-2´-deoxyuridine) to the whole embryo at the time of electroporation. The use of green or red fluorescent protein (GFP or RFP) expression plasmids allows the experimenter to quickly determine whether the electroporation successfully targeted the auditory portion of the developing inner ear. In this method paper, representative examples of GFP electroporated specimens are illustrated; embryos were harvested 18 – 96 hr after electroporation and targeting of GFP to the pro-sensory area of the auditory organ was confirmed by RNA in situ hybridization. The method paper also provides an optimized protocol for the use of the thymidine analog EdU to analyze cell proliferation; an example of an EdU based cell proliferation assay that combines immuno-labeling and click EdU chemistry is provided.
Introduction
Despite differences in morphology and cellular patterning between the mammalian and avian auditory sensory organ, the molecular factors and pathways responsible for sensory HC development are thought to be evolutionarily conserved 1-3. The basilar papilla, which houses the auditory HCs and their surrounding SCs, develops as an outpocketing of the inner ear otocyst. Early on a pool of HC and SC progenitors is specified within the otic placode/otic cup neural-sensory competent domain (NSD). Fate-mapping data provide evidence that neuronal and sensory lineages are linked and arise from the NSD located in the antero-ventral region of the otic cup or otocyst in mice and chicken 4,5. First, neuroblasts delaminate from the NSD to give rise to the neurons of the auditory-vestibular ganglion, which eventually split into auditory and vestibular ganglia. These neurons innervate the sensory HCs of the inner ear and nuclei in the brainstem. The cells that remain in the NSD are thought to give rise to various sensory patches, including the auditory sensory organ, consisting of the mechano-transducing sensory HCs and their associated SCs.
In both the chick and murine, auditory organ sensory progenitor cell-cycle exit and HC differentiation occur in opposing gradients. In chick, auditory progenitor cell-cycle exit starts around ~E5 and progresses from the base to the apex, and from the center to the periphery 6. One day later, HC differentiation starts in the apex and progresses to the base 7. Studying the development of the inner ear in chicken provides many technical advantages as functional mechanisms can be investigated with relative ease in ovo as opposed to manipulating embryos in utero in other model systems, which requires complex surgeries. The method described here uses in ovo micro-electroporation to target genes of interest in the presumptive basilar papilla area anterior-ventrally in the otic vesicle specifically at E4.
Electroporation in ovo is a technique that is well established and commonly used 8-14. The principle of the electroporation technique is based on the fact that nucleotides (e.g., plasmid DNA, synthetic DNA, or RNA oligonucleotides) are negatively charged. The DNA is injected into the tissue of interest. When an electric current is placed across the tissue, the current opens up transient pores in the cell walls and allows for the uptake of the DNA, as the negatively charged DNA flows toward the positive electrode (anode). For gain-of-function experiments, genes of interest are commonly subcloned into expression plasmids that contain appropriate expression cassettes for green fluorescent protein (GFP) or red fluorescent protein (RFP). For loss-of-function experiments plasmid-based dominant negative repressor constructs, or RNAi, or morpholino constructs are commonly used 15,16. To inhibit microRNA (miRNA) function miRNAs sponge constructs, which are typically plasmid based, can be used 17. The here described method allows for investigating the molecular mechanisms that control proliferation and differentiation in the auditory organ, as the in ovo micro-electroporation method can be easily combined with in ovo cell proliferation assay by adding EdU to the whole embryo.
The electroporation and addition of EdU is performed at E4 in the otic vesicle, which is one day before the onset of cell-cycle exit in the auditory organ. Analysis of the auditory organ is typically performed 18 – 96 hr after electroporation at E5 (onset of sensory progenitor cell-cycle exit), E6 (onset of HC differentiation), and E7 (during HC differentiation); and later up to ~E9 (end of HC differentiation). This electroporation method of gene transfer is transient lasting approximately ~4 days, because the genes of interest do not integrate into the genome, but the method is applicable for use with appropriate plasmid DNA-based expression vectors, which do have the ability to integrate into the genome, such as Tol2-mediated gene transfer 11. With this method robust GFP or RFP expression lasts ~4 days in the cochlea, after which point the signals fade, yet provide an ample time window to study the intricate development of the auditory organ. This in ovo gene transfer method is novel and allows to specifically target the presumptive auditory organ at E4, which is optimal for investigations that focus on HC development in the auditory organ. It is a good addition to alternative methods, which electroporate at much younger developmental stages 11,12 or compared to the use of in vitro basilar papillae explant cultures 18.
Protocol
The eggs and unhatched embryos are cared for and treated ethically and humanely. All protocols for unhatched embryo use were approved by the Animal Care and Use Committee at the Johns Hopkins School of Medicine, Baltimore, Maryland.
1. Eggs and Preparation of Expression Constructs
- Eggs:
- Incubate 3 – 4 dozen fertilized chicken eggs (Gallus gallus) lying flat on their sides at 37 °C.
- After 24 hr of incubation, pull 3 cc of albumen with a syringe from the round back end of the egg and seal the hole with clear tape. This creates an air chamber between the embryo and the eggshell, allowing for ease of access to the embryo without the embryo sticking to the shell when cutting the window for access on top of the egg 19.
- At 117 hr of incubation time, stage the embryos according to Hamburger and Hamilton 20 developmental stages at E4 (HH24-25) (See Figure 1D).
- Incubate 3 – 4 dozen fertilized chicken eggs (Gallus gallus) lying flat on their sides at 37 °C.
- Expression Constructs:
- Prepare the plasmid DNA using reagents and the protocol provided by the manufacturer of a commercially available preparation kit. At the final step of preparation, elute the DNA into 500 µl TE (Tris-EDTA) buffer provided in the kit into a 1.5 ml tube.
- Ethanol precipitate the DNA to concentrate it by adding 50 µl of 3 M Sodium Acetate and 1 ml of 100% Ethanol to the tube. Place the tube at -20 °C O/N.
- Centrifuge the tube for 30 min at 14,000 rpm at 4 °C. Remove the supernatant and air-dry the pellet for 10 min at RT. Dissolve the pellet in 15 µl TE buffer, which should yield a concentration of ~4 µg/µl.
NOTE: The control expression constructs used here are pMES-IRES-GFP10, which is driven by a chicken β-actin promoter, or pCI-H2B-IRES-RFP10, which is driven by the CMV promoter.
2. Chicken In Ovo Micro-electroporation and In Ovo Cell Proliferation Assay
- In Ovo Micro-injection of Expression Construct and Micro-electroporation:
- Pull the glass capillary tubes into fine needles using a micro-pipette puller with the following optimized parameters for a 2.5 x 2.5 mm Box Platinum Heating Filament: Pressure = 500, Heat = 600 °C, Pull = 64, Velocity = 105, and Time = 150 msec (see Table 1).
- Set up the Left- and Right-handed micro-manipulator stages flanking the microscope (see Figure 1A). The Right-handed micro-manipulator holds the glass capillary needle and the Left-handed micro-manipulator holds the electrodes (Figure 1A).
- Prepare the finely pulled glass capillary needle for micro-injection by cutting the tip of the needle with a forceps while working under the microscope.
- Fill the needle manually by hand using the micro-manipulator with 2.5 µl of plasmid DNA tinted with 0.1% Fast Green while working under the microscope.
- Place the egg lying on its side within a mold/egg holding device (see Figure 1A). Cut a round window on top of the egg using scissors 19 (Figure 1A).
- While working under the microscope, carefully open the two membranes overlaying the embryo using forceps.
- While working under the microscope, deliver approximately ~0.5 µl of plasmid DNA to the right otic vesicle lumen by micro-injection manually using the right hand by using the micro-manipulator and by micro-electroporation.
- To do so, micro-inject the right otic vesicle lumen with the DNA with the Right-hand micro-manipulator (Figure 1B-D) while at the same time using the left hand holding a forcep to steady the head of the embryo, because the head of the embryo dips at stage E4. Do not micro-inject past the otic vesicle lumen, because this will damage the otic vesicle.
- Immediately after micro-injection, while working under the microscope, use the Left-hand micro-manipulator to place the positive (anode) 2 mm platinum electrode anterior-ventral to the right otic vesicle and the negative 2 mm electrode (cathode) in parallel 1 mm apart (see Figure 1 C). Deliver 4 pulses at 12 V with 100 msec duration and 200 msec spacing. The left otic vesicle serves as an internal untreated control.
NOTE: Clean the electrodes in between electroporations with a cotton-tipped swab dipped in 1x PBS.
- In Ovo Cell Proliferation Assay:
- Immediately after electroporation add 50 μl of 0.25 mg/ml EdU in 1x PBS by manually dropping the 50 μl EdU solution onto the whole embryo in ovo using a pipette. Seal the eggs with tape and return to the incubator for 18 - 96 hr.
- Carefully remove the tape and check the embryos for fluorescent GFP or RFP signal within the otic vesicle after 18 – 24 hr using a standard fluorescent microscope (see Figure 1 E-E'). If fluorescent signal is present, at this point harvest the embryos for analyses or reseal with tape and return it to the incubator for harvesting and analyses at later stages (see Figure 1 F-P'').
NOTE: Expression of GFP signal with the pMES-IRES-GFP construct is evident 6 – 7 hr after electroporation 10.
3. Embryo Harvesting and Tissue Processing for RNA In Situ hybridization and Immunohistochemistry
- Harvest the embryos using forceps. Rinse the embryo in cold 1x PBS. Remove the heads of the embryos, by cutting the head by its neck using forceps, and place the heads into 4% paraformaldehyde O/N at 4 °C. Puncture the brain using a forcep to allow penetration of the 4% paraformaldehyde inside the head.
- Dehydrate the heads in 30% sucrose (in 1x PBS) O/N at 4 °C.
- Mount the heads in cryoprotective medium by rapid freezing in a slurry of dry ice and methylbutane (2-Methylbutane). Alternatively, rapid freeze the heads in cryoprotective medium with liquid nitrogen. Store the mounted heads at -80 °C.
- Cryosection the heads into 12 µm thick tissue sections and collect all the inner ear-containing tissue sections onto superfrosted microscope glass slides.
- Perform standard RNA in situ hybridization and immunohistochemistry as previously described 4,21, and analyze for EdU cell proliferation following the protocol provided by the manufacturer of the kit.
NOTE: Hair cells can be identified using rabbit polyclonal α-MyosinVIIa (Myo7a) antibody (1:1,000, Proteus Biosciences) and fluorescently-labeled secondary antibody (1:250, Goat anti-Rabbit AlexaFluor 488; Invitrogen).
4. Image Capture and Processing
- Take images with digital imaging equipment. Image immunohistochemistry results with a standard fluorescent microscope and results of the in situ using a standard wide-field microscope (ranging from 5x to 40x in magnification).
- Process the images using image processing software.
Representative Results
In this method paper plasmid DNA consisting of green fluorescent protein (GFP) expression cassettes was targeted into the developing chicken basilar papilla (BP) with optimized parameters of 12 V and 4 pulses with 100 msec pulse duration and intervals of 200 msec, yielding a ~50% embryo survival rate and efficiency of plasmid-DNA targeting into the BP. Fluorescent imaging of native GFP expression in developing embryos showed this method of electroporation preferentially targets the anterior-ventral aspect of the otocyst, that gives rise to the BP (Figure 1 E-E'; white arrow) and GFP expression can be followed over a time course of up to approximately ~4 days (Figure 1 E-H). RNA in situ hybridization (ISH) was used to determine whether the electroporation successfully targeted the auditory portion of the developing inner ear. To determine whether GFP was taken up by auditory sensory progenitors, ISH experiments were performed on adjacent BP sections. Within the developing BP sensory progenitors were identified by their expression of Sox2 (Figure 1 K and N) and Lunatic fringe (Lfng) (Figure 1 J). These experiments demonstrate that sensory progenitors throughout the developing BP, as shown for the base (Figure 1 I; black bracket) and apex (Figure 1 L; black bracket), were successfully targeted and express GFP after 68 hr of electroporation (Figure 1 I and L; black brackets). In addition, GFP was expressed outside the sensory domain in non-sensory epithelial cells (Figure 1 I and L; red arrows) and in the auditory ganglion (Figure 1I and L; ag, yellow arrows) marked by Neurod1 expression (Figure 1M). In a typical experiment GFP expression in targeted cells persisted for up to ~4 days (Figure 1 H); this allows the investigator to analyze the spatial and temporal pattern of sensory progenitor cell-cycle withdrawal and differentiation in the developing BP. To determine the proliferative behavior of sensory progenitors, the thymidine analog EdU was added at the time of electroporation at E4. Four days later, EdU incorporation in the developing BP was analyzed in tissue sections using "click" chemistry. Immuno-labeling for the HC-specific protein MyosinVIIa (Myo7a) was used to identify differentiating HCs in the developing BP. In the apical portion of the BP Myo7a+ HCs were frequently EdU+ (Figure 1 P-P'; P'', white arrows), whereas in the base only few Myo7a+ HCs had incorporated EdU (Figure 1 O-O'; O'', white arrows), suggesting that at the time of EdU addition at E4, sensory progenitors in the base are already largely post mitotic. These results confirm previous studies of opposing gradients of cell-cycle exit and HC differentiation in the chick BP 6,7.
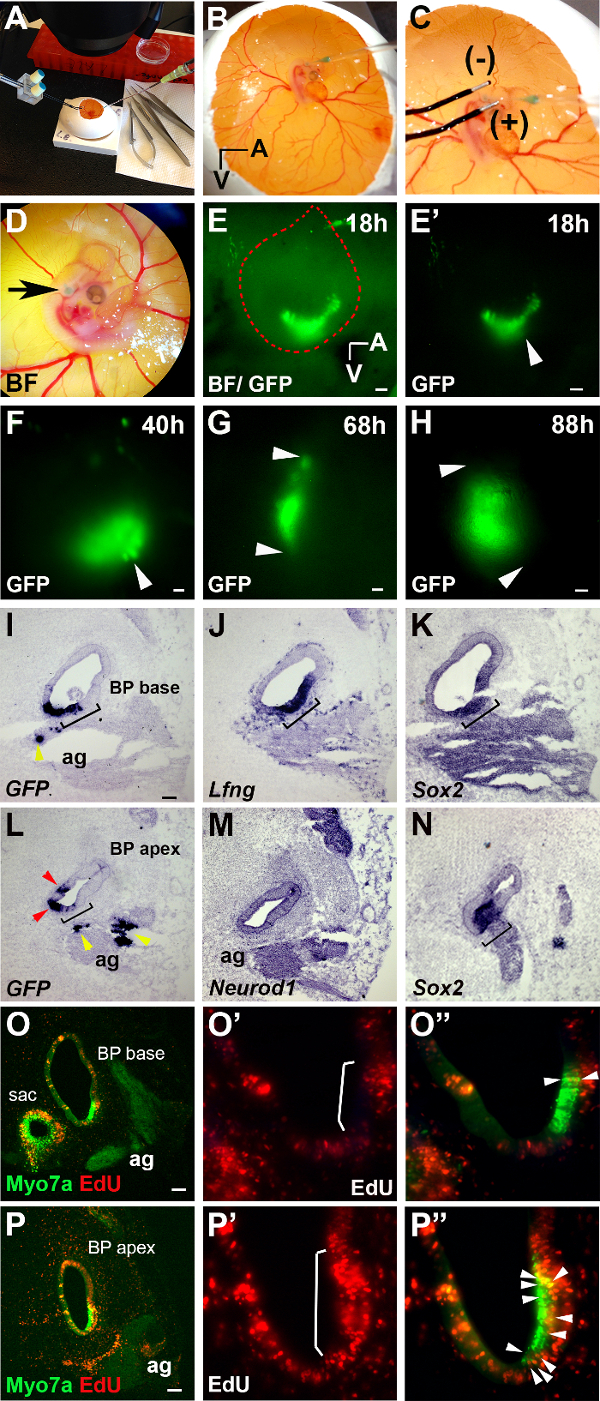
Figure 1. In Ovo Electroporation & In Ovo Cell Proliferation Assay. (A-D) Brightfield images of electroporation method. In ovo micro-injection into the right otic vesicle (B) and placement of electrodes after micro-injection targeting the anterior-ventral area in the otic vesicle (C). Embryo at stage HH24-25 after micro-injection and electroporation (D; green otic vesicle, black arrow). (E-H) Green fluorescent protein (GFP) expression after electroporation. (E) Brightfield image and GFP expression in the otic vesicle (outlined in red) at 18 hr. (E') GFP expression at 18 hr (otic vesicle, white arrow). (F-H) White arrows point to GFP expression at 40 hr (F, otic vesicle), 68 hr (G, BP), and at 88 hr (H, BP). (I-N) Adjacent cochlear tissue-sections of the BP probed for GFP, Lfng,Sox2, and Neurod1 transcripts by RNA in situ hybridization at 68 hr. Lfng (J, black bracket) and Sox2 (K and N, black bracket) mark the sensory epithelium and Neurod1 (M) marks the auditory ganglion (ag). GFP is expressed within sensory (I and L, black bracket) and non-sensory tissue (I and L, red arrows), and the auditory ganglion (I and L, yellow arrows). (O-P'') Fluorescent images of MyosinVIIa (Myo7a)/EdU labeling. (O'-O'' and P'-P'') Higher magnifications of O and P. (O' and P') More EdU labeling is present within the sensory epithelium at the apex (P', white bracket) than at the base (O', white bracket). O'' and P'') Merged fluorescent images, white arrows point to Myo7a(+)/EdU(+) hair cells at the apex (P'') and the base (O''). More EdU(+) cells are present within the auditory ganglion at the apex (P, ag) than at the base (O, ag). (sac; saccule in O). (A, Anterior and V, Ventral in panels B and E). Scale bars 100 µm. Please click here to view a larger version of this figure.
Discussion
The here described method of in ovo micro-electroporation is optimized for gene transfer into the developing auditory organ. It is compatible with plasmid DNA-based expression vectors typically used to manipulate gene function/expression. The timing of electroporation at E4 is optimal for investigations that focus on HC development in the auditory organ. The most critical steps are micro-injecting the DNA into the otic vesicle lumen without going too deep with the needle and damaging the otic vesicle (see Fig. 1A-D), targeting the DNA into the anterior-ventral domain of the otic vesicle by placing the electrodes accordingly (see Fig. 1C), and delivering the optimized electroporation parameters of 4 pulses at 12 V. These are optimized electroporation parameters and optimized concentration of plasmid DNA (~3.5 - 4 µg/µl) for specifically targeting the presumptive auditory organ at E4. Initially we started out conducting electroporations at 6 V, which yielded no fluorescent signals. The voltage was incrementally increased by using 8 V and 10 V, with 12 V being optimal. Similarly, the number of pulses to deliver and the pulse durations and spacing of pulses were optimized. At 12 V, 4 pulses with 100 msec duration and 200 msec spacing is ideal. These parameters are not only ideal for obtaining efficient gene transfer at E4, but are also ideal for an embryo survival rate of approximately ~50%. If no fluorescent signal is obtained within 24 hr of electroporation, the following should be considered: 1.) Make sure that the plasmid DNA concentration is at least 3.5 µg/µl. 2.) Adjust the electroporation parameter by 2 V, for example to 10 or 14 V. As to obtaining E4 (HH24-25) stage embryos within 117 hr of incubation time (see Fig. 1D), the incubator temperature for the eggs should be optimized between 37 – 39 °C with more or fewer hours of incubation time needed than 117 hr depending on the temperature used, because there are variations in holding and running temperatures using different incubators among different laboratories. Various thymidine analogs including BrdU (5-Bromo-2′-deoxyuridine) or EdU can be used to study in ovo cell proliferation. However, the use of either compound has to be optimized for each developmental stage as too much of the compound proves toxic for cell/embryo viability and too little of the compound proves ineffective. This method describes the optimized use of EdU at a concentration of 0.25 mg/ml applied as 50 μl onto the whole embryo in ovo specifically for studying cell proliferation and cell-cycle-exit from E4 onward.
In ovo electroporation as a method of gene manipulation has some limitations. A relative large number of eggs are required for electroporations at E4 with appropriate control constructs and for the experimental conditions. This is due to a proportion of eggs, approximately ~27%, being unusable for electroporation due to a combination of lack of fertilization, developmental defects, and embryonic death. Another important factor to consider is survival rate of the embryos after electroporation. The survival rate is approximately ~50 %. This is due to the fact that older embryos at E4 do not survive well after being manipulated; younger embryos are more resilient and tolerate being manipulated better. Expressions of the genes of interest after electroporation at E4 are focal and localized throughout the entire cochlear duct including non-sensory and sensory tissue as well as the auditory ganglion. The reason for the more focal and localized expression is because the target of the electroporation is the presumptive, undeveloped auditory organ at E4, before the chicken auditory organ starts to grow out at E5 and elongates into its characteristic sickle-shaped form by ~E9 1. Lastly, the method is transient where expression signals only remain for ~4 days, because the genes of interest used here do not integrate into the genome. However, the method can be used with appropriate plasmid-based expression constructs that do have the ability to integrate into the genome.
Mastering the electroporation method requires some practice, trained eyes and hands, and expertise in working with chicken embryos. The references provided for inner ear development and chicken developmental atlases serve as good guides and are good starting points for a novice. After mastering the method with these optimized electroporation parameters, the method can be used for manipulations in the closed otic vesicle from E2 to ~E4.5. Although for electroporating at E2 and E3, 10 V should be used instead of 12 V. For electroporation at E1 targeting the otic placode and otic cup, customized platinum electrodes with modified electroporation parameters can be used 10. The earlier manipulation can be used to study earlier events in otic development including inner ear neurogenesis and the development of vestibular sensory organs 8-10. In addition to studying the development of the auditory organ this method also lends itself to study the development of the innervating auditory ganglion. Analysis of GFP expression in electroporated specimens revealed that targeting specifically the anterior-ventral otic vesicle not only targets the sensory domain within the neural-sensory competent (NSD) epithelium but also targets the neuronal population that reside within the NSD. Afferent neurons innervating the vestibular and auditory sensory organs are primarily of otic placodal origin. Neurogenesis occurs over an extended time period approximately from ~E1.5 - E4.5 in the chick, where neuroblasts, undergoing intense cell proliferation continuously delaminate from the NSD epithelium of the otic cup (~E1.5) and otic vesicle (~E2.5 - E4.5) and populate as differentiating neurons the cochlear-vestibular ganglion (CVG; VIII cranial nerve) 8,10,13,22.
Moreover, with some modifications vestibular organs can be targeted with the method presented. For example, the presumptive anterior crista can be targeted at E3, which lies on the dorsal anterior tip of the anterior-ventral NSD 9. Electroporating at E4 targeting the anterior-ventral otic vesicle yields focal yet robust GFP transcript expressions within sensory and non-sensory tissue in all of the sensory vestibular organs, namely the cristae, and utricluar and saccular maculae (data not shown).
In conclusion, the in ovo method presented here is specific for conducting gain-and loss-of functions starting at E4 and for studying proliferation and differentiation in the developing chicken auditory organ. Moreover, this method can be easily adapted for studying other inner ear developmental phenomena, including inner ear neurogenesis and the development of vestibular organs.
Divulgations
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Doris K. Wu for expression plasmids and in situ probes, the Johns Hopkins University Center for Sensory Biology imaging facility and the Center for Hearing and Balance. This work was supported by NIDCD Grant T32 DC000023 to L.E.
Materials
| Sterile 1x PBS pH 7.4 | gibco | 10010-023 |
| Fast Green FCF Powder | Sigma | F7252-5G |
| EdU Powder | Invitrogen | E10187 |
| Click-IT EdU Alexa Fluor 555 Imaging Kit | Invitrogen | C10338 |
| HiSpeed Plasmid Midi Kit (25) | Qiagen | 12643 |
| ECM 830 ElectroSquarePorator | BTX Harvard Apparatus | |
| Banana to Micrograbber Cable Kit | BTX Harvard Apparatus | 45-0216 |
| Right Handed & Left Handed Micromanipulators | World Precision Instruments Inc. | M3301R & M3301L |
| Two 12 mm Magnetic Holding Device Stages with 7 inch vertical posts | World Precision Instruments Inc. | M10 |
| Metal Steel Base Plate 12×24 inch | World Precision Instruments Inc. | 5479 |
| Scissors for Eggs 12 cm long curved, 12 mm extrafine blades; Spring Scissors | World Precision Instruments Inc. | 14120 |
| Micropippette Puller for pulling needles | Sutter Instrument Co. | P-97 |
| 2.5×2.5 mm Box Platinum Heating Filament | Sutter Instrument Co. | |
| Glass Capillary Tubes/Needles/No Fiber/Borosil 1 mm | FHC | 27-30-0 |
| Hamilton Glass Syringe 100 μl | Hamilton | 80601 Model 710LT |
| Mineral Oil (Heavy) for Hamilton Glass Syringe | Fisher Scientific | O122-1 |
| Polyethylene Tubing for connecting the Glass Syringe and Glass Cappillary Needles/ Non Toxic | Becton Dickinson and Company (BD) | 427420 Intramedic Clay Adams Brand |
| 3 cc Disposable Syringes | Becton Dickinson and Company (BD) | 309657 |
| Disposable 21 Gauge Needles | Becton Dickinson and Company (BD) | 305122 |
| One pair of 2 mm Platinum Electrodes | Bulldog Bio. / Nepagene | CUY611P3-2 |
| Electrode Holder | Bulldog Bio. / Nepagene | CUY580 |
| One pair of Dumont fine forceps number 5 | Fine Science Tools (FST) | |
| Matte finish invisible tape for sealing eggs | Office Depot | 520-928 |
| Cotton-Tipped Swabs | Fisher Scientific | 23-400-101 |
| Sterile filter tips |
References
- Bissonnette, J. P., Fekete, D. M. Standard atlas of the gross anatomy of the developing inner ear of the chicken. The Journal of comparative neurology. 368, 620-630 (1996).
- Cantos, R., Cole, L. K., Acampora, D., Simeone, A., Wu, D. K. Patterning of the mammalian cochlea. Proc Natl Acad Sci U S A. 97, 11707-11713 (2000).
- Whitfield, T. T. Development of the inner ear. Current opinion in genetics & development. 32, 112-118 (2015).
- Raft, S., et al. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 134, 4405-4415 (2007).
- Satoh, T., Fekete, D. M. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 132, 1687-1697 (2005).
- Katayama, A., Corwin, J. T. Cell production in the chicken cochlea. The Journal of comparative neurology. 281, 129-135 (1989).
- Cotanche, D. A., Sulik, K. K. The development of stereociliary bundles in the cochlear duct of chick embryos. Brain Res. 318, 181-193 (1984).
- Alsina, B., et al. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Developmental biology. 267, 119-134 (2004).
- Chang, W., et al. Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS genetics. 4, e1000050 (2008).
- Evsen, L., Sugahara, S., Uchikawa, M., Kondoh, H., Wu, D. K. Progression of neurogenesis in the inner ear requires inhibition of Sox2 transcription by neurogenin1 and neurod1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 33, 3879-3890 (2013).
- Freeman, S., Chrysostomou, E., Kawakami, K., Takahashi, Y., Daudet, N. Tol2-mediated gene transfer and in ovo electroporation of the otic placode: a powerful and versatile approach for investigating embryonic development and regeneration of the chicken inner ear. Methods in molecular biology. 916, 127-139 (2012).
- Kamaid, A., Neves, J., Giraldez, F. Id gene regulation and function in the prosensory domains of the chicken inner ear: a link between Bmp signaling and Atoh1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 30, 11426-11434 (2010).
- Neves, J., Kamaid, A., Alsina, B., Giraldez, F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. The Journal of comparative neurology. 503, 487-500 (2007).
- Neves, J., Uchikawa, M., Bigas, A., Giraldez, F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS One. 7, 30871 (2012).
- Das, R. M., et al. A robust system for RNA interference in the chicken using a modified microRNA operon. Developmental biology. 294, 554-563 (2006).
- Wu, C. Y., Hooper, R. M., Han, K., Taneyhill, L. A. Migratory neural crest cell alphaN-catenin impacts chick trigeminal ganglia formation. Developmental biology. 392, 295-307 (2014).
- Kumar, M. S., et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci U S A. 105, 3903-3908 (2008).
- Jacques, B. E., Dabdoub, A., Kelley, M. W. Fgf signaling regulates development and transdifferentiation of hair cells and supporting cells in the basilar papilla. Hearing research. 289, 27-39 (2012).
- Korn, M. J., Cramer, K. S. Windowing chicken eggs for developmental studies. Journal of visualized experiments: JoVE. , e306 (2007).
- Hamburger, V., Hamilton, H. L. A series of normal stages in the development of the chick embryo. Journal of morphology. 88, 49-92 (1951).
- Oh, S. H., Johnson, R., Wu, D. K. Differential expression of bone morphogenetic proteins in the developing vestibular and auditory sensory organs. The Journal of neuroscience : the official journal of the Society for Neuroscience. 16, 6463-6475 (1996).
- Bell, D., et al. Spatial and temporal segregation of auditory and vestibular neurons in the otic placode. Developmental biology. 322, 109-120 (2008).

