Synthesis of Zeolites Using the ADOR (Assembly-Disassembly-Organization-Reassembly) Route
Summary
A protocol for the preparation of novel zeolites by the ADOR (Assembly-Disassembly-Organization-Reassembly) synthetic route is presented.
Abstract
Zeolites are an important class of materials that have wide ranging applications such as heterogeneous catalysts and adsorbents which are dependent on their framework topology. For new applications or improvements to existing ones, new zeolites with novel pore systems are desirable. We demonstrate a method for the synthesis of novel zeolites using the ADOR route. ADOR is an acronym for Assembly, Disassembly, Organization and Reassembly. This synthetic route takes advantage of the assembly of a relatively poorly stable that which can be selectively disassembled into a layered material. The resulting layered intermediate can then be organized in different manners by careful chemical manipulation and then reassembled into zeolites with new topologies. By carefully controlling the organization step of the synthetic pathway, new zeolites with never before seen topologies are capable of being synthesized. The structures of these new zeolites are confirmed using powder X-ray diffraction and further characterized by nitrogen adsorption and scanning electron microscopy. This new synthetic pathway for zeolites demonstrates its capability to produce novel frameworks that have never been prepared by traditional zeolite synthesis techniques.
Introduction
Zeolites are a class of solids that consist of a three-dimensional open arrangement of corner-sharing tetrahedra, where the metal cation (traditionally silicon and aluminum) at the centers of the tetrahedral are surrounded by 4 oxide anions. Different arrangements of these corner-sharing tetrahedra lead to different zeolite frameworks that can possess a wide variety of pore architectures. These pore structures are able to accommodate small molecules, which leads to their applications within petrochemical, nuclear and medical fields, among others. Note that zeolite topologies and materials are given codes that identify their topology (such as UTL) or an actual material (e.g., IPC-2) — for more information please see the website of the International Zeolite Association, www.iza-online.org.
The crucial feature of zeolites is their porosity, which defines their utility by governing the amount and accessibility of the internal surface area where most of the important chemistry occurs. This in turn determines the chemical activity and selectivity of the materials. A major goal in zeolite science (and indeed in all porous material science) is to control the porosity.
Zeolites are traditionally synthesized by the hydrothermal method,1,2 which has changed little in the past 50 years. In fact, the last major advances occurred in 1961 with the introduction of quaternary ammonium salts as structure directing agents1 and in 1982 with the discovery that phosphorus could be substituted for silicon giving rise to the aluminophosphate family of materials.3 Given the great utility of zeolites, there is great interest in developing new routes to novel materials. Such a route is the recently developed ADOR strategy4–7 where a parent zeolite is Assembled, then Disassembled and the resulting species Organized in such a way as to allow final Reassembly into a new solid. This makes use of a pre-prepared zeolite that has inherent instability built into its framework, which we can exploit.8 This poor stability stems from the incorporation of hydrolytically unstable germanium that is preferentially located within the D4R (double four-ring) units that bind adjacent silica rich layers together (Figure 1). These D4R units can be selectively removed using a relatively mild treatment allowing further chemical manipulations to be performed on the intermediate layered material.4
The major difference between traditional hydrothermal synthesis and ADOR is the final method of framework formation. In hydrothermal synthesis this is a reversible process allowing the final structure to be crystalline. In the ADOR process, however, the final framework formation stage (Reassembly) is an irreversible condensation of the layers at high temperature. The key to getting highly crystalline final materials is then the Organization step, where the layered intermediates are arranged in the right relative positions to allow the irreversible condensation into new frameworks to happen as optimally as is possible.
In the following example we show how the parent zeolite, a germanosilicate with the UTL zeolite topology,9,10 can be prepared (the Assembly step) using a pre-prepared organic cation as a structure-directing agent (SDA). The key to the success of this protocol is the location of germanium in specific places in the zeolite, which allows the parent Ge-UTL to be disassembled and organized, using hydrolysis in acid to produce the layered intermediate called IPC-1P. This intermediate can then be treated in two different ways. Direct reassembly of the IPC-1P material at high temperature leads to a zeolite with the IPC-4 structure, whose topology is given the code PCR by the International Zeolite Association (IZA). However, the IPC-1P can be organized differently through intercalation of a silicon-containing species between the layers. We call the result of this manipulation IPC-2P. High temperature treatment of this intercalated and organized IPC-2P material leads to a new zeolite called IPC-2, whose topology is given the IZA code OKO. The difference between the OKO (IPC-2) and PCR (IPC-4) topologies is that IPC-2 contains silica subunits (a single four ring, S4R) between UTL-like layers whereas IPC-4 has no S4R units.
The zeolites are characterized by X-ray diffraction, N2adsorption and Energy Dispersive X-ray Analysis using a scanning electron microscope.
Protocol
Caution: Please consult all relevant material safety data sheets (MSDS) and carry out a risk assessment of all procedures before use. Several of the chemicals used in this synthesis procedure are acutely toxic and carcinogenic. Please use all appropriate safety procedures during the duration of these procedures including engineering controls (fume hood) and personal protective equipment (safety glasses, lab coat and suitable chemical resistant gloves).
1. Preparation of the Structure-directing Agent for the Synthesis of UTL
- Dissolve 5.68 g of sodium hydroxide in 140 ml of distilled water. Add 30.66 g of 1,4-dibromobutane to the solution and stir.
- Heat the solution to reflux (oil bath at 110 °C).
- Add drop wise (~1 drop per second) 16.07 g of (2R,6S)-2,6-dimethylpiperidine and continue to reflux for a further 12 hr.
- Cool to RT and then chill in an ice bath.
- Dissolve 50 g of sodium hydroxide in 75 ml of distilled water to prepare a 40 wt% solution. Cool on ice. Add 70 ml of ice cooled 40 wt% sodium hydroxide solution to the chilled solution above.
- Filter the white precipitate and dissolve in minimal chloroform.
- Extract the aqueous residue with chloroform (three 200 ml portions). Combine the chloroform portions and dry using ~20 g of anhydrous sodium sulfate.
- Remove the sodium sulfate by filtration using filter paper, and wash with chloroform (50 ml).
- Partially evaporate the chloroform using a rotary evaporator until a white precipitate starts to form. Remove from the rotary evaporator and add diethyl ether until no further precipitation is visible.
- Recover the white precipitate by filtration using filter paper in a funnel and wash with diethyl ether (50 ml).
- Dry the white precipitate at 60 °C O/N to yield the salt (6R,10S)-6,10-dimethyl-5-azoniaspiro[4,5]decane bromide.
- Dissolve 25.0 g of (6R,10S)-6,10-dimethyl-5-azoniaspiro[4,5]decane bromide in 50 ml of distilled water.
- Exchange the bromide anion for hydroxide anion by using a hydroxide ion-exchange resin.
- Add 25.0 g of a hydroxide ion-exchange resin to the bromide salt solution and stir for 12 hr.
- Filter the solution with filter paper and re-expose the filtrate to 25.0 g of hydroxide ion-exchange resin under stirring for 12 hr. Repeat exposure to hydroxide ion-exchange resin until a silver nitrate test for halogens returns a negative result.
Note: The silver nitrate test is a very sensitive test for bromide ions.- Acidify 0.25 ml of the product solution with 2 ml dilute nitric acid (1.0 M). Add 2-3 drops of silver nitrate solution (0.05 M) to the product solution. If a precipitate forms then repeat exposure to the hydroxide ion-exchange resin and retest until no precipitate can be seen.
- Confirm the concentration of hydroxide by titration with 0.1 M (which is equivalent to 0.1 N) hydrochloric acid using phenolphthalein solution as an indicator. Using a burette, slowly add small aliquots of 0.1 M hydrochloric acid to a small portion of known volume of the product solution until the phenolphthalein indicator just changes color (i.e., the end point is reached). The total number of moles of hydrochloric acid added is equal to the number of moles of hydroxide in the initial solution.
- Dilute the solution with distilled water to give a concentration of 0.625 M in hydroxide.
2. Preparation of Parent Ge-UTL Zeolite
- Dissolve 1.08 g of germanium dioxide into 15 ml of a solution of the structure directing agent (6R,10S)-6,10-dimethyl-5-azoniaspiro[4,5]decane hydroxide (concentration of 0.625 M).
- Add portion-wise 1.246 g of fumed silicon dioxide to the above solution and stir for a further 30 min until a homogeneous solution is formed.
Note: The resulting gel has a molar composition of 0.8 SiO2: 0.4 GeO2: 0.4 ROH: 30 H2O, where ROH is the structure directing agent. - Transfer the gel to a polytetrafluoroethylene-lined autoclave (30 ml capacity). Then place in an oven and heat to 175 °C for 10 days.
- After 10 days, remove the autoclave from the oven, and allow to cool naturally to RT. Recover the white zeolite product by filtration. Wash with copious amounts of water (~200 ml). Dry the zeolite at 70 °C O/N.
- Remove the structure directing agent from the pores of the zeolite by heating the zeolite to 550 °C at a rate of 1 °C min–1, held at 550 °C for 6 hr before being cooled to RT at a rate of 2 °C min–1.
- Acquire a powder X-ray diffraction spectrum to confirm the structure using manufacturer's protocol.
Note: The powder X-ray diffraction pattern should match that given for UTL in Figure 2. - Acquire a N2 adsorption isotherm to confirm the porosity using manufacturer's protocol.
- Acquire elemental analysis using Energy Dispersive X-ray Spectroscopy using manufacturer's protocol.
- Store the calcined zeolite in a dry inert atmosphere to prevent hydrolysis of the material.
3. Hydrolysis of Ge-UTL to Form IPC-1P
- Add 1.0 g of calcined zeolite to 160 ml of a 0.1 M hydrochloric acid solution.
- Heat this mixture at 95 °C for 18 hr, cool to RT and recover the solid by filtration using a filter paper. Wash with copious amounts of water (~300 ml) and dry at 70 °C O/N.
- Acquire a powder X-ray diffraction spectrum to confirm the structure of IPC-1P using manufacturer's protocol.
Note: The powder X-ray diffraction pattern should match that given for IPC-1P in Figure 2. The dried product is designated IPC-1P and is stored for further use.
4. Preparation of IPC-4 Zeolite
- Place 0.5 g of IPC-1P in a ceramic crucible and heat to 575 °C at a heating rate of 1 °C min–1, hold at 575 °C for 6 hr before being cooled to RT at a rate of 2 °C min–1.
- Acquire a powder X-ray diffraction spectrum to confirm the structure using manufacturer's protocol.
Note: The powder X-ray diffraction pattern should match that given for IPC-4 in Figure 2. - Acquire a N2 adsorption isotherm to confirm the porosity using manufacturer's protocol.
- Acquire elemental analysis using Energy Dispersive X-ray Spectroscopy using manufacturer's protocol. This will give information on how much Ge remains in the structure.
5. Preparation of IPC-2 Zeolite
- Add 0.5 g of IPC-1P to 10 ml of 1.0 M nitric acid solution.
- Add 0.1 g of diethoxydimethylsilane (DEDMS) to the solution.
- Transfer the solution to a polytetrafluoroethylene-lined autoclave and heat in an oven at 175 °C for 18 hr.
- Remove the autoclave from the oven and allow to cool naturally to RT.
- Recover the white product by filtration, wash with copious amounts of water (~100 ml) and dry at 70 °C O/N.
- Acquire a powder X-ray diffraction spectrum to confirm the structure. The powder X-ray diffraction pattern should match that given for IPC-2P in Figure 2.
- Place the product in a ceramic crucible and heat to 575 °C at a heating rate of 1 °C min–1, hold at 575 °C for 6 hr before being cooled to RT at a rate of 2 °C min–1.
- Acquire a powder X-ray diffraction spectrum to confirm the structure using manufacturer's protocol.
Note: The diffraction pattern should match that given for IPC-2 in Figure 2. - Acquire a N2 adsorption isotherm to confirm the porosity using manufacturer's protocol.
- Acquire elemental analysis using Energy Dispersive X-ray Spectroscopy using manufacturer's protocol.
Representative Results
Powder X-ray diffraction patterns (Figure 2) were collected for all materials produced, including the intermediate layer phases IPC-1P and IPC-2P. Powder X-ray diffraction is the primary technique used to determine the nature of the zeolite phases present. Note that the crystal structures of IPC-1P and IPC-2P are not fully characterized, as the materials are always somewhat disordered. However, each powder diffraction pattern can be used as a 'fingerprint' for the phase of interest. The most important feature to look for is the position of the peaks in the pattern, which gives information on the unit cell size. Each of the zeolites (and the intermediates) has a different unit cell size and so the peak positions in each collected diffraction pattern are diagnostic for the presence of that particular phase, and should match the positions of the reference patterns shown in Figure 2. In particular the position of the most intense peak is the first thing to look for. If the position of this main peak matches the position in the reference patterns then one should look to see if the other peaks also match. If the diffractometer used is well aligned and maintained then this match should be relatively good. Extra peaks present in the sample X-ray diffraction patterns that are not present in the respective pattern in Figure 2 indicate that the prepared sample is not phase pure. The intensities of the peaks in the diffraction pattern are not important for the phase identification procedure, and they can differ between the sample and reference patterns because of differences in instrumentation so can be ignored. Intensities only become important when completing full structural studies to get information on atomic positions, which is not necessary in this study.
While X-ray diffraction is the primary method of structural analysis, nitrogen adsorption isotherms (Figure 3) can also be used to characterize the product zeolites. This experimental method first requires that any molecules (usually water) that are present in the pores of the channel are removed by heating the sample, usually under a vacuum. Then the sample is cooled, usually to 77 K, and small amounts of nitrogen gas are administered to the system and either gravimetric or volumetric measurements used to determine how much nitrogen has been adsorbed by the sample. The amount of nitrogen adsorbed is plotted against the pressure of gas to give the isotherms shown in Figure 3. A successful synthesis will show isotherms of a similar shape to those show in Figure 1. In the best situations the total amount adsorbed will be greatest for the parent UTL sample, with the amount being lower for IPC-2 and lowest for IPC-4. This matches the change in pore sizes. From this data it is also possible to obtain surface areas using the BET equation (Table 1).11
Elemental analysis is another technique that can be used to determine the extent to which germanium has been removed from the product. Any suitable chemical analysis technique can be used, but we have used energy dispersive X-ray analysis (EDX) using a scanning electron microscope to ascertain the composition of the parent Ge-UTL zeolite and the final zeolites IPC-2 and -4 (Table 1).
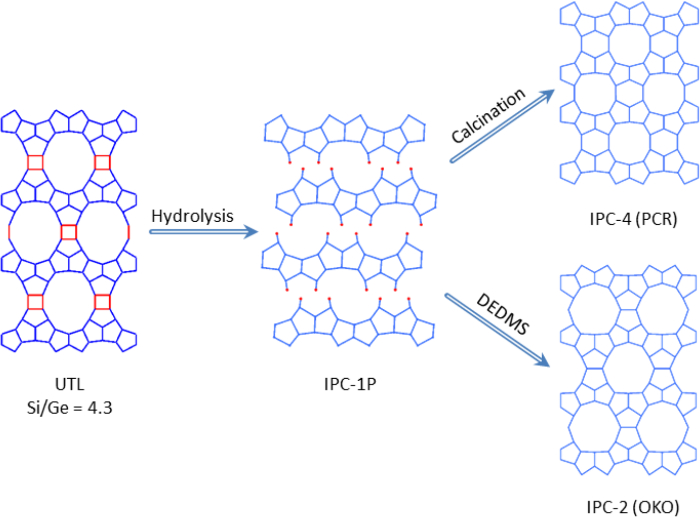
Figure 1. Proposed schematic of mechanism from parent zeolite UTL to final zeolites IPC-2 & -4. D4R unit of parent UTL is highlighted in red. Please click here to view a larger version of this figure.
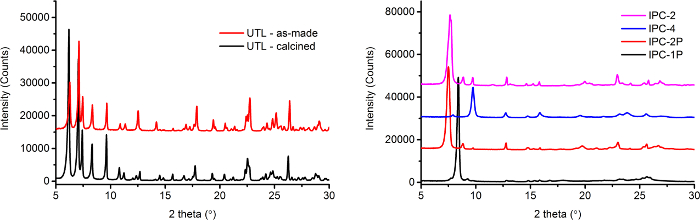
Figure 2. Powder X-ray diffraction patterns of the parent 'as-made' and calcined UTL zeolite (left) and the intermediates and final zeolites (right). IPC-2P is the product of intercalating the layered IPC-1P with DEMDA prior to calcination. Please click here to view a larger version of this figure.
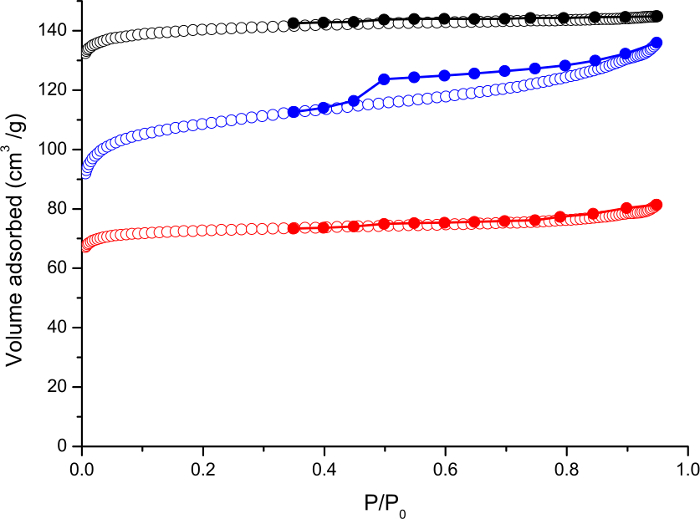
Figure 3. Nitrogen adsorption isotherms recorded at 77 K for UTL (black), IPC-2 (blue) and IPC-4 (red). Adsorption isotherm is shown as unfilled shape and the desorption isotherm as filled shape. This figure is kindly reproduced by permission of Nature Publishing Group from reference 4. Please click here to view a larger version of this figure.
| Zeolite | BET Surface Area (m2/g) | Si:Ge Ratio |
| Ge-UTL | 541.3 ± 1.1 | 5.8 |
| IPC-2 | 334 ± 1.0 | >500 |
| IPC-4 | 236 ± 0.7 | 90 |
Table 1. Porosity values and elemental composition of zeolites.
Discussion
A full description of the actual mechanism of the ADOR process is beyond the scope of this paper, but can be found in the published papers cited.3,5,8 However, it is worth expanding on the potential importance of the process. The ADOR method of zeolite preparation differs considerably from traditional methods of zeolite synthesis in the manner in which the final material is prepared. The most important consequence of this is that materials prepared using the ADOR process have the potential to be fundamentally different from traditionally made zeolites. In particular there is scope to use the ADOR method to prepare materials that are energetically distinct. The theory behind this is described in reference 8.
The control over porosity is another area where the ADOR method shows different properties to traditional methods.13 In particular, it is possible to prepare a whole series of zeolites with continuously tuneable porosity, which has not so far been possible for zeolites prepared using hydrothermal synthesis. The modification to enable the series is in step 3 of the process described above. By altering the concentration of the acid used from 0.1 M all the way up to 6 M (and even beyond) one can tailor the nature of the final material. Full details of how this can be achieved is given in reference 13. This is both a great opportunity and a risk. Sometimes if the concentration of the acid used, the temperature and the time left to react are not optimum the resultant materials show a diffraction pattern where the position of the most intense peak does not match those shown in Figure 2. However, in such a situation this can be recognized by comparing the powder X-ray patterns from the experiment with those described in reference 13.
The critical steps in the protocol that ensure that a successful outcome is achieved are those dealing with the manipulations. Firstly it is important that any solutions in contact with the layered intermediates are not alkaline, as this promotes dissolution of silica, especially at high temperature. Secondly, the irreversible final step of the ADOR process is the key factor, and so the proper organization of the material (steps 3.2 and 5.2) is crucial for success of the process. As described above, time and acidity are both important variables in the process and so ensuring that these steps are optimized is extremely important.
As described above there is a requirement that the parent zeolite is a germanosilicate with the germanium located in specific places in the structure. This will limit the number of zeolites that can be used as the parent. Zeolite UTL is the only material that has been significantly explored as a parent. However, there are early indications that other parents might be successfully applied to the process, but further work is required in this area.
To ensure the ADOR method works, great care must be taken in the manipulations after the disassembly step to ensure that the layers of the intermediate IPC-1P do not dissolve or undergo significant rearrangement. It is also important to get the acidity, time and temperature of the reaction conditions right to optimize the final products. Such fine control over reaction conditions can be rather confusing in the first instance, and is a major driving force behind our wish to have a video description of the procedure.
In conclusion, this procedure describes how the ADOR method of zeolite synthesis can be applied to the germanosilicate with the UTL framework structure to form two different zeolites, IPC-2 (OKO) and IPC-4 (PCR).
Divulgations
The authors have nothing to disclose.
Acknowledgements
R.E.M. thanks the Royal Society and the E.P.S.R.C. (Grants EP/L014475/1, EP/K025112/1 and EP/K005499/1) for funding work in this area. J.Č. acknowledges the Czech Science Foundation for the project of the Centre of Excellence (P106/12/G015) and the European Union Seventh Framework Programme (FP7/ 2007--2013) under grant agreement n°604307. The authors would like to thank P. Chlubná-Eliášová, W.J. Roth and P. Nachtigall for enlightening discussions.
Materials
| Sodium hydroxide | Fisher Chemical | S/4920/53 | 99% |
| 1,4-dibromobutane | Aldrich | 140805-500G | 99% |
| (2R,6S)-2,6-dimethylpiperidine | Aldrich | 41470-100ML | >99% |
| Paraffin oil | Fisher Chemical | P/0320/17 | |
| Chloroform | Fisher Chemical | C/4920/17 | >99% |
| Sodium sulfate (anhydrous) | Fisher Chemical | S/6600/60 | >99% |
| Diethyl ether | Sigma Aldrich | 24002-2.5L | >99.5% |
| Ambersep 900-OH | Acros Organics | 301340025 | |
| Hydrochloric acid, 0.1N | Fluka | 318965-500ML | |
| Phenolphthalein | Sigma Aldrich | 105945-50G | ACS Reagent |
| Silver nitrate | Ames Goldsmith | ||
| Germanium dioxide | Alfa Aesar | 11155 | 100.00% |
| fumed silica (Cab-o-sil M-5) | Acros Organics | 403731500 | |
References
- Cundy, C. S., Cox, P. A. The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chem. Rev. 103 (3), 663-701 (2003).
- Cundy, C. S., Cox, P. A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Micropor. Mesopor. Mater. 82 (1-2), 1-78 (2005).
- Wilson, S. T., Lok, B. M., Messina, C. A., Cannan, T. R., Flanigen, E. Aluminophosphate molecular-sieves – a new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 104 (4), 1146-1147 (1982).
- Roth, W. J., et al. A family of zeolites with controlled pore size prepared using a top-down method. Nat. Chem. 5 (7), 628-633 (2013).
- Roth, W. J., Nachtigall, P., Morris, R. E., Cejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 114 (9), 4807-4837 (2014).
- Chlubná, P., et al. 3D to 2D Routes to Ultrathin and Expanded Zeolitic Materials. Chem. Mater. 25 (4), 542-547 (2013).
- Chlubná-Eliášová, P., et al. The Assembly-Disassembly-Organization-Reassembly Mechanism for 3D-2D-3D. Transformation of Germanosilicate IWW Zeolite. Angew. Chem. Int. Ed. 53 (27), 7048-7052 (2014).
- Morris, R. E., Čejka, J. Exploiting chemically selective weakness in solids as a route to new porous materials. Nat. Chem. 7 (5), 381-388 (2015).
- Paillaud, J. L., Harbuzaru, B., Patarin, J., Bats, N. Extra-large-pore zeolites with two-dimensional channels formed by 14 and 12 rings. Science. 304 (5673), 990-992 (2004).
- Corma, A., Diaz-Cabanas, M. J., Rey, F., Nicolooulas, S., Boulahya, K. ITQ-15: The first ultralarge pore zeolite with a bi-directional pore system formed by intersecting 14- and 12-ring channels, and its catalytic implications. Chem. Comm. , 1356-1357 (2004).
- Rouquerol, J., Llewellyn, P., Rouquerol, F. Is the BET equation applicable to microporous adsorbents?. Stud. Surf. Sci. Catal. 160, 49-56 (2006).
- Trachta, M., Bludsky, O., Cejka, J., Morris, R. E., Nachtigall, P. From Double-Four-Ring Germanosilicates to New Zeolites: In Silico Investigation. Chemphyschem. 15 (14), 2972-2976 (2014).
- Wheatley, P., et al. Zeolites with continuously tuneable porosity. Angew. Chem. Int. Ed. 53 (48), 13210-13214 (2014).

