High-resolution Fiber-optic Microendoscopy for in situ Cellular Imaging
Summary
In many biological and clinical situations it is advantageous to study cellular processes as they evolve in their native microenvironment. Here we describe the assembly and use of a low-cost fiber-optic microscope which can provide real time imaging in cell culture, animal studies, and clinical patient studies.
Abstract
Many biological and clinical studies require the longitudinal study and analysis of morphology and function with cellular level resolution. Traditionally, multiple experiments are run in parallel, with individual samples removed from the study at sequential time points for evaluation by light microscopy. Several intravital techniques have been developed, with confocal, multiphoton, and second harmonic microscopy all demonstrating their ability to be used for imaging in situ 1. With these systems, however, the required infrastructure is complex and expensive, involving scanning laser systems and complex light sources. Here we present a protocol for the design and assembly of a high-resolution microendoscope which can be built in a day using off-the-shelf components for under US$5,000. The platform offers flexibility in terms of image resolution, field-of-view, and operating wavelength, and we describe how these parameters can be easily modified to meet the specific needs of the end user.
We and others have explored the use of the high-resolution microendoscope (HRME) in in vitro cell culture 2-5, in excised 6 and living animal tissues 2,5, and in human tissues in vivo 2,7. Users have reported the use of several different fluorescent contrast agents, including proflavine 2-4, benzoporphyrin-derivative monoacid ring A (BPD-MA) 5, and fluoroscein 6,7, all of which have received full, or investigational approval from the FDA for use in human subjects. High-resolution microendoscopy, in the form described here, may appeal to a wide range of researchers working in the basic and clinical sciences. The technique offers an effective and economical approach which complements traditional benchtop microscopy, by enabling the user to perform high-resolution, longitudinal imaging in situ.
Protocol
1. Microendoscope Assembly
The high-resolution microendoscope described here (figure 1a) should be considered as a base configuration with several variations possible in assembly and application. We describe in detail here an embodiment of the platform which is designed to be used with proflavine as a fluorescent contrast agent. Proflavine is a bright nuclear stain with peak absorption and emission wavelengths of 445 nm and 515 nm respectively. The use of other contrast agents will require the user to select excitation, emission, and dichroic filters appropriately. Several elements of the high-resolution microendoscope are generic and may be obtained from multiple vendors. For example, optomechanical positioning components are available from Thorlabs, Newport, Linos among others. Compact CCD cameras are available from companies including Point Grey Research, Prosilica, and Retiga; camera sensitivity should be chosen with consideration of the brightness of the fluorophore to be used, as well as the desired frame rate. High-power light emitting diodes (LEDs) may be obtained from Luxeon, Cree, and Nichia among others. Fiber-optic bundles are available from Sumitomo, Fujikura, and Schott. In selecting components for a specific application, the user should consider the inherent relationships involved in fluorescence microscopy between fluorophore concentration, photobleaching, illumination intensity, camera sensitivity, gain, and exposure time.
- Connect the 6″ cage rods to the 1.5″ cage rods, to form a pair of 7.5″ long rods. Screw these rods into one face of the fold mirror unit. Screw the 0.5″ rods into the opposite face. Slide the cage cube onto the cage rods, via the two lower through holes. Screw the 2″ rods into the side face of the cage cube (figure 1b).
- Attach the camera to a cage plate by using a C-mount to SM1 adapter. Secure the cage plate on the 0.5″ cage rods, flush with the face of the fold mirror unit.
- Insert the “tube” lens into a 3″ long lens tube and secure the lens with a retaining ring. The focal length of the lens should be selected such that the cores of the fiber-optic bundle are sampled by at least two pixels when projected onto the camera. Drop the emission filter into the tube on top of the first retaining ring, and add another ring to secure the filter in place. Observe the filter orientation convention indicated by the filter manufacturer. Screw the lens tube into the side of the cage cube nearest to the CCD camera. Note that in figure 1c, this lens and filter are shown without the 3″ lens tube for clarity.
- Connect the camera to a computer and view the image on screen. Direct the cage assembly at a distant object and slide the cage cube along the rails until an image appears in focus. Lock down the cage cube at this location. (This is a simple method of ensuring that the tube lens will form a focused image when combined with the infinity-corrected objective lens).
- Insert the dichroic mirror into the holder and place at 45° in the cage cube.
- Screw the objective lens, via a RMS to SM1 thread adapter and an adjustable lens tube, into the face of the cage cube opposite the tube lens holder. A z-translator may be used in place of the adjustable lens tube for easier focusing. Screw an SMA connector into a cage plate and mount this on the rods, approximately at the working distance of the objective lens.
- Mount an LED on a cage plate and slide onto the end of the 2″ rods. Add a lens to a 0.5″ lens tube such that when this tube is screwed into the side of the cage cube, it will form an image of the LED that fills the back aperture of the objective lens. This (Kohler illumination) configuration will ensure that the proximal face of the fiber-optic bundle is uniformly illuminated. Add the excitation filter to the 0.5″ lens tube and secure in place with a retaining ring (figure 1c).
- Attach a SMA connector to a fiber-optic bundle. Screw the SMA connectorized bundle into the SMA receptacle mounted on the cage rods. Direct the distal end of the bundle towards a broadband light source (fluorescent lighting will suffice) and observe the image of the bundle proximal face on the CCD camera. Adjust the position of the objective lens by screwing in or out of the cage cube until the fiber bundle image appears in focus. The individual cores should be clearly visible (Figure 2).
2. GRIN Lens Assembly
The spatial resolution of the microendoscope can be increased by attaching a micro-lens or lens assembly to the distal tip of the fiber bundle. These optics are configured such that instead of placing the bundle tip directly on to the tissue, the tip is imaged onto the tissue surface with demagnification, thereby increasing the spatial sampling frequency imposed by the light-guiding cores of the fiber bundle. The degree of demagnification corresponds to the increase in spatial resolution, and at the same time, to a proportionate decrease in field-of-view. Gradient index (GRIN) lens components are compatible with fiber-optics and are available from GrinTech, NSG, Schott, among others, and can be directly bonded to the distal tip of a fiber bundle.
- Select a GRIN lens with the desired magnification and working distance for your application. Ensure that the diameter of the lens exceeds that of the fiber bundle you plan to work with. We recommend that the fiber bundle and GRIN lens be mounted on separate 3-axis manual positioning stages under a low power microscope or stereoscope for accurate alignment prior to bonding.
- Place a drop of optical adhesive (eg. Norland UV curing adhesive) on either the lens or bundle face. Bring the two components into contact by using the manual positioners. Expose the interface to UV light for the dose recommended by the manufacturer.
- To protect the GRIN lens and the bonded interface, a short length of aluminum capillary tubing (Small Parts Inc.) can be used to enclose the joint. Slide the tubing over the joint and secure in place with epoxy. Heat shrink tubing can be used to finish the assembly.
3. Microendoscope Imaging
- Apply the contrast agent to the cells or tissue to be imaged. With proflavine (0.01% w/v in PBS), in vitro imaging of cells in culture can be performed by brief incubation (< 1 minute) and thorough rinsing. Imaging of ex vivo tissue specimens or in vivo tissues is possible following topical application of the dye. Uptake of proflavine under these conditions has been found to be near-instantaneous, with imaging possible within a few seconds and lasting for several minutes.
- Place the fiber optic bundle in light contact with the sample to be imaged. When imaging cells in culture or ex vivo tissue specimens, we recommend mounting the distal end of the fiber bundle in a secure fixture with manual positioning stages on XYZ axes for stability during imaging.
4. Representative Results:
When assembled correctly, the microendoscope will operate as an epi-fluorescence microscope, relayed through a coherent fiber-optic bundle. For optimal imaging results, attention should be paid to ensuring that three key conditions are met:
- The proximal face of the fiber bundle should be imaged onto the CCD camera without defocus, in order to achieve the full resolution of the system. Figure 2a,b,c demonstrate a portion of a fiber bundle imaged with poor focus, slight defocus, and good focus, respectively. The optimum focus is found by adjusting the axial position of the objective lens relative to the bundle face.
- The proximal face of the fiber bundle should be uniformly illuminated over its full diameter (field-of-view). As described in the Protocol Text (1.7), this is achieved by configuring the illumination optics for Kohler illumination. Figure 2d shows an image acquired when a uniform fluorescent sample is illuminated in this preferred configuration, with Figure 2e demonstrating the corresponding result under critical illumination. In the latter case, the structure of the LED is imaged onto the fiber bundle face, resulting in the appearance of this unwanted pattern superimposed upon the true sample structure.
- Both the proximal and distal faces of the fiber bundle should be clean and free of scratches and chips. Figure 2f demonstrates the presence of debris attached to the bundle face, with damage in the form of a small chip at 5 o’clock on the fiber perimeter. Debris can be removed from the bundle faces by cleaning in the same fashion as conventional fiber-optic connectors, with lens paper and isopropanol, or standard fiber-optic cleaning tools. If either end of the fiber bundle is scratched or chipped, or if the bundle breaks along its length, the face may be polished flat by standard manual, or mechanical polishing techniques. We recommend 12-15 μm lapping paper for an initial coarse polish, with a final polish on 0.5-1.0 μm paper.
Figure 3a demonstrates imaging of 1483 cells in vitro, following labeling with proflavine and light placement of the bare fiber bundle on the sample. Figure 3b demonstrates the improvement in spatial resolution and reduction in field-of-view provided by a 2.5x GRIN lens bonded to the bundle tip. Movie 1 demonstrates in vivo imaging of the mammary fat pad in a mouse model. Here, a fiber bundle with 0.5 mm outer diameter (330 μm field-of-view) was passed through a 21-gauge needle and advanced into the tissue. Fat cells are clearly visible, with motion due to the cardiac cycle apparent in this acquisition at 15 frames per second. Figure 3c demonstrates imaging of the oral mucosa in a healthy human volunteer, this time using a larger fiber bundle with 1.5 mm outer diameter (1.4 mm field-of-view). In all examples shown, proflavine was used as a nuclear labeling fluorescent contrast agent.
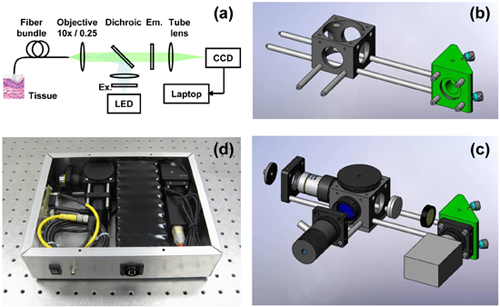
Figure 1. Assembling the high-resolution microendoscope (HRME). (a) Schematic diagram of the HRME system. (b) Assembly of the main optomechanical support structure. (c) Addition of optical elements, illumination LED, and CCD camera. (d) Photograph of the HRME system, packaged in a 10″ x 8″ x 2.5″ enclosure.
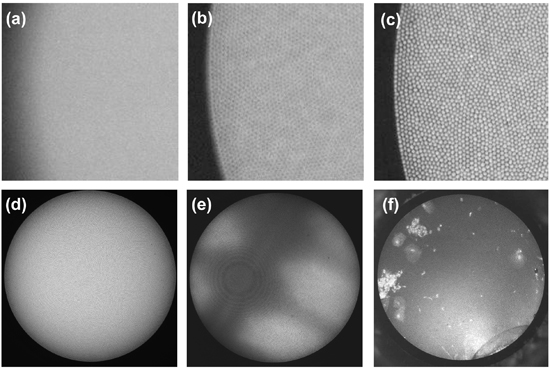
Figure 2. Setting up the HRME. Examples of imaging with the fiber-optic bundle in (a) poor focus, (b) close to good focus, (c) ideal focus. In (d), a uniform fluorescent target at the bundle’s distal tip is imaged under Kohler (uniform) illumination. (e) A uniform fluorescent target imaged under critical illumination, with the source structure apparent on the object. (f) Loose tissue and cells can stick to the fiber bundle face, which is also prone to minor damage at its periphery.
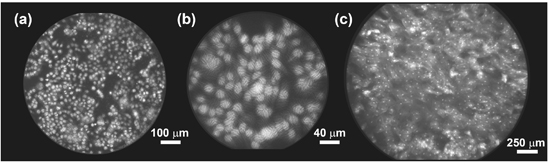
Figure 3. Imaging with the HRME. (a) 1483 cells in vitro, imaged with a bare fiber bundle (IGN-08/30) following labeling with proflavine 0.01% (w/v). (b) The same 1483 cell culture as shown in (a), imaged with a fiber bundle with 2.5x GRIN lens attached. (c) Image of normal human oral mucosa in vivo, following topical application of proflavine 0.01% (w/v).
Movie 1. Imaging the mammary fat pad of a mouse via insertion of a 450 μm outer diameter fiber bundle within the lumen of a 21-gauge needle passed into the tissue. Proflavine 0.01% (w/v) was delivered to the imaging site through the same needle prior to insertion of the imaging fiber. Click here to watch video
Discussion
The high-resolution microendoscopy technique described here provides researchers in the basic biomedical and clinical research areas with a flexible, robust, and cost-effective method for visualizing cellular detail in situ. We have described a protocol for assembling the imaging system and demonstrated its use in cell culture in vitro, and in animal, and human tissues in vivo. While the imaging results presented here used proflavine as a fluorescent contrast agent, other groups have demonstrated versions of the system with LED illumination wavelengths and filters chosen to match excitation / emission spectra of other dyes 5-7.
Resolution and field-of-view are initially determined by the core-to-core spacing and imaging diameter of the fiber-optic bundle. We have used bundles with approximately 4 μm core-core spacing, and imaging diameters of 330 μm (movie 1), 720 μm (Figure 2, Figure 3a,b), and 1400 μm (Figure 3c). The smaller bundles can be passed through narrower gauge needles and are significantly more flexible than the larger fibers. We and others 8 have, in some cases, noted the appearance of autofluorescence emissions from the fiber bundle itself. When attempting to excite fluorophores at UV wavelengths, or collect emission in the red spectral range, attention should be paid to the level of fiber bundle autofluorescence contributing to the overall measured signal.
While most of the high-resolution microendoscopy work reported to-date has used a bare fiber bundle, additional magnification can be provided by use of GRIN lenses bonded to the distal tip. GRIN lenses offer a straightforward and economical way to increase spatial resolution, though their susceptibility to optical aberrations and limited NA is well recognized. If GRIN lens performance is inadequate for a particular application, hybrid GRIN / spherical lens objectives 9 or miniature objective lens assemblies 10-11 can be employed.
The high-resolution microendoscope described here essentially operates as a wide-field epi-fluorescence microscope; therefore no optical sectioning (as in confocal or nonlinear microscopy) is to be expected. In our experience, using 455 nm excitation light and topical proflavine as a contrast agent, light is primarily collected from a depth corresponding to a few cell layers.
This protocol ought to enable the reader to assemble the high-resolution microendoscope on the benchtop, with a compact footprint of 10″ x 8″. If desired, the system may be enclosed in a box and the electrical components (LED and camera) powered by a battery pack (Figure 1d). Many compact cameras can be powered by the IEEE-1394 (Firewire) and USB ports of the host computer.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This research was partly funded by the National Institutes of Health, grant R01 EB007594, the Department of Defense Breast Cancer Research Program, proposal BCO74699P7, and the Susan G. Komen Foundation grant 26152/98188972.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| CCD camera | Point Grey Research | GRAS-14S5M | ||
| LED | Thorlabs | M455L2 | Selected for use with proflavine – other fluorophores may require different parts | |
| Excitation filter | Semrock | 452/45 | Selected for use with proflavine – other fluorophores may require different parts | |
| Emission filter | Semrock | 550/88 | Selected for use with proflavine – other fluorophores may require different parts | |
| Dichroic mirror | Chroma | 485 DCLP | Selected for use with proflavine – other fluorophores may require different parts | |
| Objective lens | Thorlabs (Olympus) | RMS 10X | ||
| Tube lens | Thorlabs | AC-254-150-A1 | Select focal length to achieve required magnification to CCD | |
| Condenser lens | Thorlabs | ACL2520 | ||
| Cage cube unit | Thorlabs | C6W, B1C, B3C, B5C, SM1CP2 | ||
| Cage rods and plates | Thorlabs | ER05 (x4), ER1.5 (x2), ER2 (x2), ER6 (x2), CP02 (x3) | ||
| Fold mirror unit | Thorlabs | KCB1, PF10-03-G01 | ||
| Lens tubes | Thorlabs | SM1L05, SM1L30, SM1V05 (or SM1Z) | ||
| Adapters / couplers | Thorlabs | SM1A3, SM1A9, SM1T2 (x2) | ||
| SMA connectors | Thorlabs | SM1SMA, 11040A | ||
| LED driver | Thorlabs | LEDD1B TPS001 | ||
| Fiber optic bundle | Sumitomo | IGN-08/30 | Larger or smaller bundles are available (Sumitomo / Fujikura) |
References
- Pierce, M. C., Javier, D. J., Richards-Kortum, R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int. J. Cancer. 123, 1979-1990 (2008).
- Muldoon, T. J., Pierce, M. C., Nida, D. L., Williams, M. D., Gillenwater, A., Richards-Kortum, R. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt. Express. 15, 16413-16423 (2007).
- Muldoon, T. J., Anandasabapathy, S., Maru, D., Richards-Kortum, R. High-resolution imaging in Barrett’s esophagus: a novel, low-cost endoscopic microscope. Gastrointest. Endosc. 68, 737-744 (2008).
- Muldoon, T. J., Thekkek, N., Roblyer, D., Maru, D., Harpaz, N., Potack, J., Anandasabapathy, S., Richards-Kortum, R. Evaluation of quantitative image analysis criteria for the high-resolution microendoscopic detection of neoplasia in Barrett’s esophagus. J. Biomed. Opt. 15, 026027-026027 (2010).
- Zhong, W., Celli, J. P., Rizvi, I., Mai, Z., Spring, B. Q., Yun, S. H., Hasan, T. In vivo high-resolution fluorescence microendoscopy for ovarian cancer detection and treatment monitoring. Br. J. Cancer. 101, 2015-2022 (2009).
- Dubaj, V., Mazzolini, A., Wood, A., Harris, M. Optic fibre bundle contact imaging probe employing a laser scanning confocal microscope. J. Microsc. 207, 108-117 (2002).
- Dromard, T., Ravaine, V., Ravaine, S., Lévêque, J. -. L., Sojic, N. Remote in vivo imaging of human skin corneocytes by means of an optical fiber bundle. Rev. Sci. Inst. 78, 053709-05 (2007).
- Udovich, J. A., Kirkpatrick, N. D., Kano, A., Tanbakuchi, A., Utzinger, U., Gmitro, A. F. Spectral background and transmission characteristics of fiber optic imaging bundles. Appl. Opt. 47, 4560-4568 (2008).
- Barretto, R. P. J., Messerschmidt, B., Schnitzer, M. J. In vivo fluorescence imaging with high-resolution microlenses. Nat. Methods. 6, 511-512 (2009).
- Rouse, A. R., Kano, A., Udovich, J. A., Kroto, S. M., Gmitro, A. F. Design and demonstration of a miniature catheter for a confocal microendoscope. Appl. Opt. 43, 5763-5771 (2004).
- Kester, R. T., Christenson, T., Richards-Kortum, R., Tkaczyk, T. S. Low cost, high performance, self-aligning miniature optical systems. Appl. Opt. 48, 3375-3384 (2009).

