Extracellular Recording of Electrical Activity in the Drosophila Central Nervous System
Abstract
Source: Swale, D. R., et al. Electrophysiological Recording of The Central Nervous System Activity of Third-Instar Drosophila Melanogaster. J. Vis. Exp. (2018)
The video demonstrates the extracellular recording of neuronal activity from the central nervous system (CNS) of Drosophila larvae. The transected CNS is positioned in a recording chamber, and the electrical activity from a descending nerve is recorded to establish the baseline firing frequency. A test agent is then introduced, and the change in firing frequency is recorded to determine the agent's excitatory or inhibitory effect.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Dissect and Prepare the Larval Drosophila CNS
NOTE: Methods for larval CNS dissection are clearly illustrated in Hafer and Schedl, but these previously published methods reduce the length of the descending neurons that are important for measuring spike frequency. Here, an additional method is outlined to excise the larval CNS that maintains long, intact descending neurons.
- Saline preparation.
- Prepare the dissection and recording saline to the following in mM: 157 NaCl, 3 KCl, 2 CaCl2, 4 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES); titrate pH to 7.25.
- Dissect the larval Drosophila CNS.
- Identify and extract a wandering third-instar Drosophila melanogaster from the culture vial and place into 200 µL of saline (Figure 1A).
- Grasp the mouth hooks with a pair of fine forceps and grasp the abdomen of the maggot with a second pair of forceps (Figure 1B).
NOTE: Do not apply too much pressure to the abdomen as this can result in tearing of the thin cuticular layer of the maggot. - Gently pull the mouth hooks and abdomen in different directions to separate the caudal end of the maggot from the head region and expose the viscera of the maggot (Figure 1C).
NOTE: The CNS will be intertwined with the trachea and digestive tract. Do not cut the CNS away from any tissue to prevent cutting the descending peripheral nerves. - Tease the CNS out of the digestive tract and trachea with forceps (Figure 1D).
- If necessary, disrupt the blood brain barrier by manually transecting the CNS posterior to the cerebral lobes with Vannas spring scissors.
NOTE: This should be done based on the physiochemical properties of the chemical being used. The red line in Figure 2A is the suggested transection point and the transected CNS with intact descending peripheral nerve trunks shown in Figure 2B. The transected CNS is ready for experimentation after completion of step 1.2.5.
2. Extracellular Recording of Drosophila CNS.
- Prepare the CNS for recording.
- Pull a glass pipette electrode from borosilicate glass capillaries to a resistance of 5-15 mΩ.
- Insert the transected CNS into the wax chamber that contains 200 µL of saline. Clamp an uncoated insect pin with the alligator clip soldered to the ground wire and insert the pin into the saline to complete the circuit.
- Using the micromanipulators, orient the electrode to the caudal end of the transected CNS. Eliminate recording of background noise by adjusting the threshold level in the acquisition/analysis software prior to contacting the peripheral nerve trunks.
- Draw any convenient peripheral nerves into the suction electrode by applying slight negative pressure on the syringe.
NOTE: The baseline firing frequency is correlated to the number of neurons drawn into the electrode where more neurons oftentimes result in increased resistance of the electrode.
- Begin extracellular recording of CNS descending nerve activity.
- Start the recording on the data acquisition software and monitor the baseline firing frequency. Allow the firing rate to equilibrate for 5 min prior to collecting baseline firing rate data.
- Discard the preparation and recording if the pattern of firing of control treatment is not a bursting pattern similar to that shown in Figure 3A.
NOTE: Altered pattern of firing suggests abnormal or unstable activity of the central pattern generator, which can alter neural function. - After 5 min, add 200 µL of saline + vehicle to bring the total volume of the chamber to 400 µL to begin recording control firing rates.
- After baseline has been established (3-5 min), withdraw 200 µL of saline and add 200 µL of the experimental agent solubilized in saline.
NOTE: Dimethyl sulfoxide (DMSO) is the recommended solvent for lipophilic compounds and should not exceed 0.1% v/v. - Apply drug concentrations in a serial manner (low to high concentrations) and record each concentration for a select period of time (determined by the investigator based on the chemical properties of the drug). Label this time point of drug application in the acquisition/analysis software by including a comment that includes the drug and the final concentration.
NOTE: Firing activity of the CNS will decline by approximately 10-20% after 30-50 min after the dissection and therefore, appropriate untreated controls should be performed, and drug treatments should not exceed this time period.
Representative Results
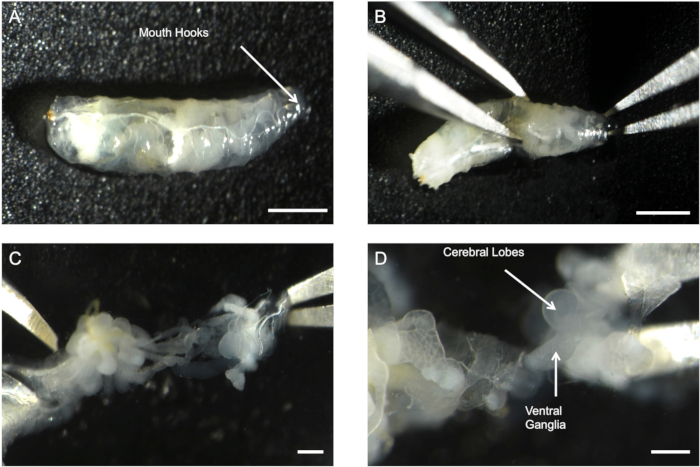
Figure 1: Method for excising the CNS from third-instar maggots. (A) Intact maggot submerged in 200 µL of saline. The arrow indicates the mouth hooks that are used for separation of the body wall. (B) Two pairs of forceps are placed in the middle of the maggot and on the mouth hooks to begin separation of the body wall. (C) Body wall is separated by applying slight and continuous pressure to expose the viscera. (D) CNS is clearly visible (white arrows) and is occasionally intertwined with the viscera. The scale bar represents 1000 µm, 750 µm, 500 µm, and 200 µm for panels A, B, C, and D, respectively.
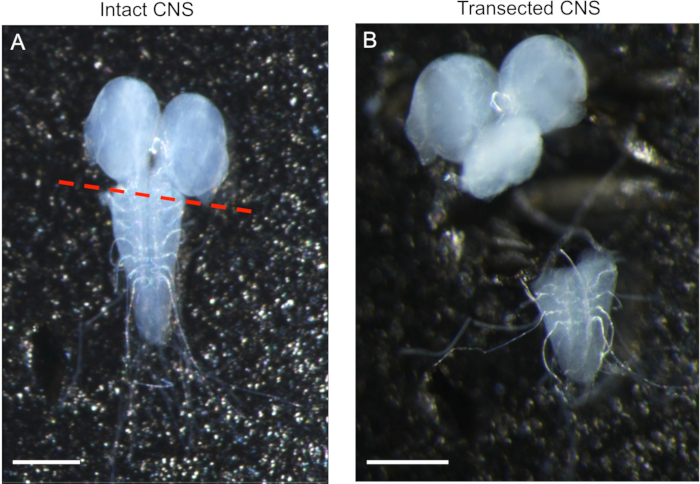
Figure 2: Disruption of the blood brain barrier by transecting the CNS. (A) Intact CNS with descending nerves clearly visible at the caudal end of the ventral ganglia. The red line indicates the location of transecting the CNS to disrupt the BBB. (B) A transected CNS with the caudal end of the ventral ganglia still exposing long descending neurons. The ventral ganglia can be discarded. The scale bar represents 200 µm for both panels.
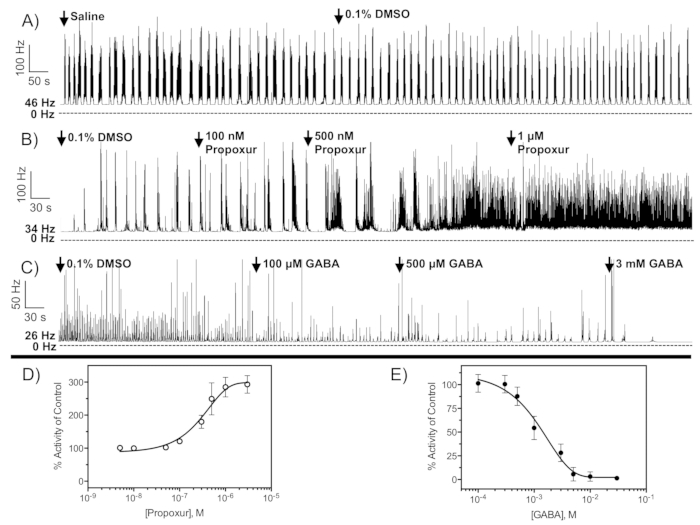
Figure 3: Neurophysiological recordings from the CNS of third-instar larvae of D. melanogaster. Representative nerve discharge traces before and after exposure to (A) DMSO, (B) propoxur, and (C) GABA. Initial firing frequencies in spikes/s (Hz) for each experiment are given to the left of each trace. Concentration-response curves for propoxur (D) and GABA (E) to CNS nerve discharge of D. melanogaster larvae from replicated recordings (n = 3-5 concentrations per curve, with each concentration replicated at least 5 times). Arrows represent point of drug application. Data points represent mean percent increase of baseline firing rate and error bars represent standard deviation. When error bars are absent, it is because they are smaller than the size of the symbol.
Divulgations
The authors have nothing to disclose.
Materials
| Drosophila melanogaster (strain OR) | Bloomington Drosophila Stock Center | 2376 | |
| Vibration isolation table | Kinetic Systems | 9200 series | |
| Faraday Cage | Kinetic Systems | N/A | |
| Dissecting Microscope on a Boom | Nikon | SMZ800N | Multiple scopes can be used; boom stand is critical |
| AC/DC differential amplifier | ADInstruments | AM3000H | The model 1700 can be used instead of the model 3000 |
| audio monitor | ADInstruments | AM3300 | |
| Hum Bug Noise Eliminator | A-M Systems | 726300 | |
| Data Acquisition System (PowerLab) | ADInstruments | PL3504 | Multiple PowerLab models can be used. |
| Lab Chart Pro Software | ADInstruments | N/A – Online Download | |
| Fiber Optic Lights | Edmund Optics | 89-740 | Different light sources can be used, but fiber optics are the most adaptable |
| Micromanipulator | World Precision Instruments | M325 | |
| Microelectrode Holder | World Precision Instruments | MEH715 | Different models are acceptable |
| BNC cables | World Precision Instruments | multiple based on size | |
| Glass Capillaries | World Precision Instruments | PG52151-4 | |
| Microelectrode Puller | Sutter Instruments | P-1000 | Also can use Narashige PC-100 |
| Black Wax | Carolina Biological Supply | 974228 | |
| Non-coated insect pins, size #2 | Bioquip | 1208S2 | |
| Fince Forceps | Fine Science Tools | 11254-20 | |
| Vannas Spring Scissors | Fine Science Tools | 15000-03 |

