Electrophysiological Recording of Voltage Responses of Drosophila Retinal Photoreceptors to Light Stimuli
Abstract
Source: Juusola, M., et al. Electrophysiological Method for Recording Intracellular Voltage Responses of Drosophila Photoreceptors and Interneurons to Light Stimuli In Vivo. J. Vis. Exp. (2016).
This video outlines the protocol for electrophysiological recording of photoreceptor activity in the eye of an immobilized Drosophila, enabling precise measurement of voltage changes in response to light stimuli.
Protocol
1. Recording from R1-R6 Photoreceptors
- Always be grounded when operating the microelectrode amplifier (for example by touching the metal surface of the Faraday cage or antivibration table), as this precludes one from accidentally delivering a static charge to the head-stage, which could damage the circuitry.
- Illuminate the fly preparation platform pole from above with two gooseneck light guides (Figure 1A) (with the cold light source inside the Faraday cage) so that the fly-holder can be placed on the pole in the preferred position under close visual control.
- Mount the fly-holder (with the fly in it!) on the fly preparation platform pole. Rotate the fly-holder so that the fly's left eye is directly facing the investigator (Figure 1B).
- Insert the blunt reference electrode gently through the fly's ocelli into the head capsule using a small coarse micromanipulator while observing the preparation through the stereomicroscope (Figure 1C). Do not push the electrode too deep, as this can damage the fly brain.
- Alternatively, insert the reference electrode into the back of the thorax. Always ensure that the fly appears healthy (moves its antennae), and its eyes are intact, not accidentally damaged. If the preparation looks less than immaculate, prepare a new fly for the experiments.
- Drive the sharp recording microelectrode into the left eye through a petroleum jelly covered small opening. Use high magnification in the stereomicroscope and move the light guides and the focal plane so that the electrode tip location becomes apparent in 3D by its reflectance patterns.
NOTE: Figure 1D shows how the fly's head should be positioned (with respect to the angle at which the recording microelectrode enters the eye) for photoreceptor recordings. Driving the electrode into the eye without breaking it is the most difficult phase of the experiment. If the electrode tip misses the small opening in the eye, hitting the cornea, it typically breaks. - Turn on the microelectrode amplifier once both electrodes are firmly inside the preparation, in electrical contact with the fly's body fluids.
- Turn off the cold-light source (inside the Faraday cage) and unplug it from the mains. Connect its plug to the central ground to minimize ground-loop induced electrical noise and move the goose-neck light guides away so the Cardan-arm system can be freely moved around the fly. Switch off the room lights to ensure that the fly preparation is now in relative darkness.
- Measure the resistance of the recording electrode in the eye. Use only recording electrodes in which resistance is 100 – 250 MΩ.
NOTE: Achieving high-quality intracellular recordings by <70 MΩ electrode is virtually impossible. If the resistance is <80 MΩ, it is likely that the electrode tip is broken. In this case, switch off the amplifier and change the recording electrode. - Set the amplifier to current-clamp (CC) or bridge recording mode. Cancel out any arbitrary voltage difference between the recording and reference electrodes, as both are now resting in the electrically interconnected extracellular space, by setting the signal offset (recording voltage) to zero. Follow the signal offset changes using the amplifier's display readout or an oscilloscope screen.
- Wait 2-3 min for the fly eye to dark-adapt.
- Drive the recording electrode tip deeper into the eye with small 0.1 to 1-micron steps. Do this with an x-axis piezo-stepper of a remote-controlled micromanipulator or by gently rotating the fine-resolution knob of a manual manipulator.
- Stimulate the fly eye with brief (1 – 10 msec) light flashes as the recording electrode is being advanced in the tissue.
NOTE: If the recording electrode is positioned in the retina and the eye functions normally, each light flash will cause a brief and small drop in the voltage (0.2 – 5 mV hyperpolarization), called the electroretinogram (ERG). This change in the field potential of the extracellular space is caused by the retinal cells' collective response to light.
Representative Results
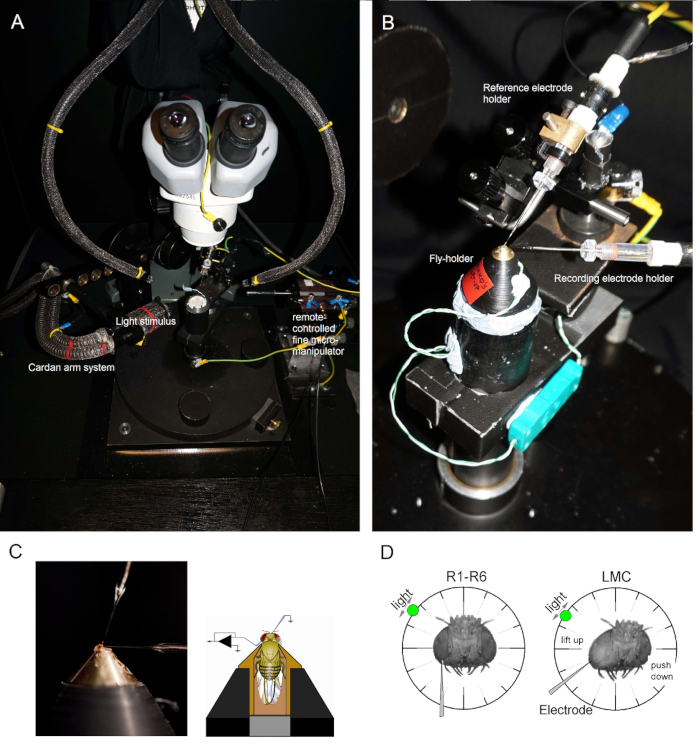
Figure 1. Positioning the Fly-holder and the Electrodes for the Experiments. (A-B) The fly-holder is placed on the recording platform that also provides temperature control via a Peltier element (A: white round platform in the center). The Cardan-arm enables exact positioning of the light stimulus at an equal distance (via x,y-rotation) around the fly, with the light source (a liquid or quartz fiber-optic bundle end) directly pointing to its eye. In many of our rigs, light stimulation is generated by LEDs (with linear current drivers) or by a monochromator. Thus, their stimuli carry a specific (band-passed) spectral content, selected between 300 – 740 nm and cover 4 – 6 log intensity unit range (as attenuated by separate neutral density filters). (C) Two microelectrodes, controlled by separate micromanipulators, are positioned in the fly head: the reference electrode (above) through the ocelli; the recording electrode (left) through the small opening in the left eye. (D) For obtaining a maximum number of photoreceptor recordings, the recording microelectrode is driven into the hole, parallel to the proboscis-ocellus axis. When the electrode tip penetrates and seals to a photoreceptor, the freely rotatable light source is fixed to the position where the cell produces the maximum voltage response to a given light stimulus. This point in space lies in the center of the cell's receptive field.
Divulgations
The authors have nothing to disclose.
Materials
| Stereo Zoom Microscope for making the fly preparation | Olympus | SZX12 DFPLFL1.6x PF eyepieces: WHN30x-H/22 | Capable of ~150X magnification with long working distance; bespoke heavy steel table mount stand |
| Stereomicroscope in the intracellular set-up | · Olympus | Olympus SZX7; eyepieces: WHN30x-H/22 | 30X eyepieces are needed for seeing the electrode tip reflections well when driving it through the small corneal hole into the eye |
| Nikon microscope | Nikon SMZ645; eyepieces: C- W30x/7 | ||
| Anti-vibration Table | Melles Griot | With metric M6 holes on the breadboard | Our bespoke rigs have a large hole drilled through the thick breadboard that lets in the fly preparation platform pole (houses a copper heatsink with electronics) from below |
| Newport | |||
| Micromanipulators | Narishige | Narishige NMN-21 | In our intracellular set-ups, different micromanipulator systems are used for driving the shap recording electrodes into the fly eye. All the listed manipulators are succesfully providing long-lasting stable recordings from Drosophila photoreceptors and LMCs. |
| Huxley Bertram | Huxley xyz-axis with fine manual control | ||
| Sensapex | Sensapex triple axis | ||
| Märzhäuser | Märzhäuser DC-3K with additional x-axis piezo stepper and MS 314 controller | ||
| Magnetic Stands | Any magnetic base with on/off switch will do | For example, to manage cables inside the Faraday cage | |
| Electrode Holders | Harvard Apparatus | ESP/W-F10N | |
| Silver Wire | World Precision Instruments | AGW1510 | 0.3 – 0.5 mm diameter; needs to be chloridized for the electrode holders |
| Fiber Optic Light Source | Many different, including Olympus | ||
| Fiber Optic Bundles | UltraFine Technology | To deliver the LED light stimulus to the Cardan arm system. We use both liquid and quartz light guides (range from UV to IR) | |
| Thorn Labs | |||
| Fly Cathing Tube | P80-50P 50ml Cent. Tube PP., Pack of 100 Pcs | Cut the conical bottom off from 50 ml Plastic Centrifuge Tube and glue a 1 ml pipette tip on it. | |
| Digital Acquisition System | National Instruments | ||
| Single-electrode current/voltage- clamp microelectrode amplifier | npi SEC-10LX | http://www.npielectronic.de/ products/amplifiers/sec-single- electrode-clamp/sec-10lx.html | Outstanding performer! |
| Head-stage | Standard (+/- 150 nA) | For npi SEC-10LX | |
| LED light sources and drivers | 2-channel OptoLED (Cairn Research Ltd., UK) | Many of our stimulus systems are in-house built | |
| Self-designed and constructed | |||
| Acquisition and Analyses Software | Many companies to choose from | Biosyst; custom written Matlab- based system for experimental and theoretical work in the Juusola laboratory | |
| Personal Computer or Mac | Ensure that PC or Mac is compatible with data acquisition system and software | ||
| Cardan arm system | Self-designed and constructed | Providing accurate x,y,z-positioning of the light stimuli | |
| Peltier temperature control system | Self-designed and constructed | ||
| Faraday Cage | Self-constructed | Electromagnetic noise shielding | |
| Filamented Borosilicate Glass Capillaries | Outer diameter: 1 mm | ||
| Inner diameter: 0.5 – 0.7 mm | |||
| Filamented Quartz Glass Capillaries | Outer diameter: 1 mm | ||
| Inner diameter: 0.5 – 0.7 mm | |||
| Pipette Puller | Sutter Instrument Company | Model P-2000 laser Flaming/Brown Micropipette Puller | For borosilicate reference electrodes, use the preset program #11 (patch electrodes): Heat = 350; Filament = 4; Velocity 36; Delay = 200).1.2.1). For borosilicate recording electrodes, use the preset program #12 (this typically pulls good conventional sharps for photoreceptor recordings): Heat = 355; Filament = 4; Velocity 50; Delay = 225; Pull = 150. For LMC recordings, which require electrodes with finer tips, these values need to be adjusted. For pulling quartz capillaries, P-2000 manual suggests programs for fine tipped microelectrodes. These programs’ preset parameters serve as useful starting points for systematic modifications to generate electrodes with good penetration success and low recording noise. |
| Extracellular Ringer Solution for the reference electrode | Chemicals from Fisher Scientific | 10326390, NaCl 10010310, KCl 10147753, TES 10161800, CaCl2 10159872, MgCl2 10000430, sucrose |
See the recipe in the protocol section |
| 3 M KCl solution for filling the filamented recording microelectrode | Salts from Fisher Scientific | 10010310, KCl | |
| Petroleum jelly | Vaselin |

