Dissection and Mounting of Fruit Fly Larval Brain Explants
Abstract
Source: Sheng, C., et al. Time-lapse Live Imaging and Quantification of Fast Dendritic Branch Dynamics in Developing Drosophila Neurons. J. Vis. Exp. (2019).
This video demonstrates the dissection and mounting of brain explants from Drosophila larvae. The central nervous system (CNS), attached to eye disks, is extracted from the larvae and then mounted on a glass slide in a physiological saline solution.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Dissecting and Mounting Larval Brain Explants
- Dissect larval brains in the physiological external saline solution (120 mM NaCl, 4 mM MgCl2, 3 mM KCl, 10 mM NaHCO3, 10 mM Glucose, 10 mM Sucrose, 5 mM N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (TES), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM Ca2+, pH 7.2) under a dissection microscope (4.5x magnification power) with two pairs of #5 standard tip dissection forceps (11 cm). Use one pair of forceps to hold the larval body in place and the other to carefully dissect out the brain. Preserve the eye disks, brain lobes and the ventral nerve cord. Remove attached muscles to minimize sample movements during imaging.
- Prepare a glass slide (25 x 75 x 1.0 mm3) and use a syringe to draw a square chamber with vacuum grease.
- Add 20 µL of external saline solution to the square chamber with grease barriers.
- Transfer dissected larval brains into the chamber on the glass slide using forceps. Adjust the position of the brains under the dissection scope to ensure the dorsal side faces up.
- Cover the chamber with a glass cover slip (22 x 22 x 0.15 mm3). The larval brain is now mounted on the slide within a chamber filled with the external saline solution (Figure 1B).
NOTE: Pressing gently on the coverslip confines the brains and reduces sample drifting in the subsequent imaging session.
Representative Results
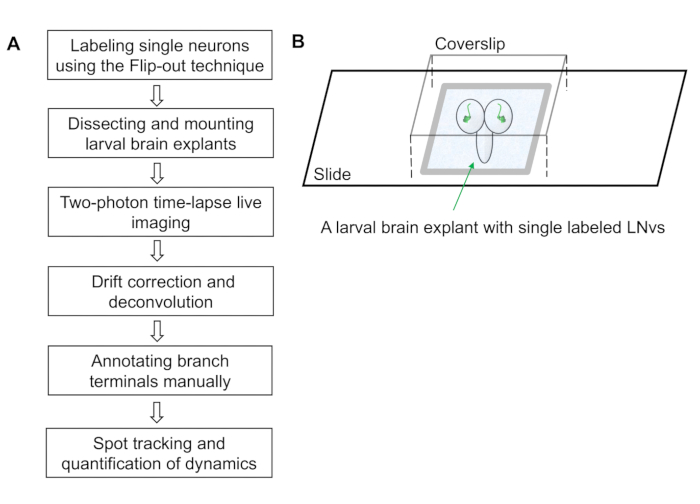
Figure 1: Workflow of the dendrite dynamics imaging and quantification protocol. (A) The protocol contains six steps covering sample preparation, image collection, image processing and semi-automated quantification of dendrite dynamics. (B) A schematic diagram illustrating an imaging chamber containing a larval brain explant mounted with the dorsal side up. The brain explant has an individually labeled ventral lateral neuron (LNv) in each lobe and is immersed in the external saline solution. The vacuum grease barriers support the weight of the coverslip and forms a small chamber that prevents the brain from moving.
Divulgations
The authors have nothing to disclose.
Materials
| high vacuum grease | Dow Corning | 79751-30 | |
| Microscope Cover Glass | Fisher Scientific | 12-544-E | |
| Superfrost Plus Microscope Slides | Fisher Scientific | 12-550-15 | |
| Reagents | |||
| Glucose | |||
| HEPES | |||
| KCl | |||
| MgCl2 | |||
| NaCl | |||
| NaHCO3 | |||
| PBS | |||
| Sucrose | |||
| TES |

