Generation of Tilted Amygdala Slices from a Mouse Brain
Abstract
Source: Bosch, D., et al. Ex Vivo Optogenetic Dissection of Fear Circuits in Brain Slices. J. Vis. Exp. (2016)
The video demonstrates the generation of tilted amygdala slices from a mouse brain. The brain is positioned at an angle on the vibratome specimen stage to obtain slices containing the amygdala and axons from the medial prefrontal cortex that form synaptic connections with the amygdala. The brain slices are maintained at the physiological temperature to preserve the tissue for further experiments.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Preparation of Acute Slices
- Prepare artificial cerebrospinal fluid (ACSF), cutting solution, tools (scissors, scalpel, forceps, spatula, Pasteur pipette), and agar blocks.
Note: 1 L of artificial cerebrospinal fluid (ACSF) is required per experiment, and is prepared by dissolving chemicals in double-distilled H2O as previously published. The cutting solution is prepared by supplementing 200 ml of ACSF with 0.87 ml of 2 M MgSO4 stock solution. - Oxygenate ACSF and cutting solution throughout the experiment with 95% O2 and 5% CO2.
- Deeply anesthetize mouse using a small animal anesthesia machine using isoflurane (3% in oxygen). Check anesthesia depth using limb withdrawal reflex before continuing.
- Decapitate mouse using large scissors and immediately chill head in ice-cold cutting solution.
- Open skull by a single midline incision from caudal to rostral and gently push pieces of skull to the sides using forceps. Rapidly remove brain by gently lifting it out of the skull using a small, rounded spatula. Cut off cerebellum with scalpel. Place brain in ice-cold cutting solution.
- For medial prefrontal cortex or mPFC injection sites: cut off anterior part of the brain (containing mPFC) using a scalpel and put in ice-cold cutting solution until slicing.
- Remove excess cutting solution with filter paper and glue the posterior part of the brain onto the vibratome stage.
- For tilted amygdala slices, glue the brain on an agar block (4%) cut at a 35° angle (Figure 1D, middle). For coronal amygdala slices, medial geniculate nucleus/ posterior intralaminar thalamic nucleus (MGm/PIN) injection sites and mPFC injection sites, glue the brain directly on stage (Figure 1D, left and right). Place an additional agar block behind brain for stability while slicing.
- Place the stage in cutting chamber with ice-cold oxygenated cutting solution that is maintained at 4 °C using a cooling unit. Prepare acute slices of the amygdala (320 µm) using a sapphire blade. Place slices in an interface chamber supplied with oxygenated ACSF at room temperature (RT).
- After preparation of acute amygdala slices, place the interface chamber in a water bath at 36 °C to recover slices for 35 – 45 min. Subsequently, return the interface chamber to RT.
- During commencement of step 1.10, cut slices of the injection sites as described before for acute amygdala slices (steps 1.8 – 1.9). Recovery of slices in the water bath is not required here. Optional: To quickly estimate injection site location, observe slices on a stereoscope equipped with a fluorescent lamp and appropriate filter sets.
Representative Results
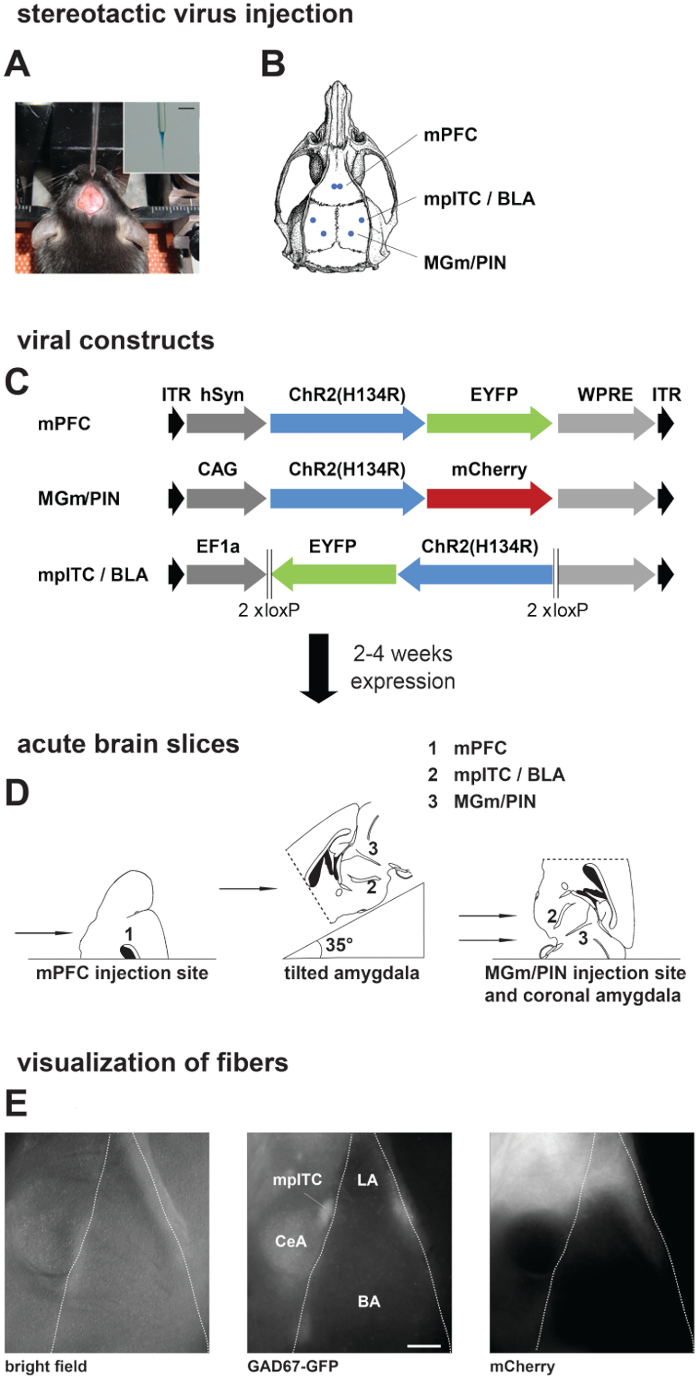
Figure 1. Stereotactic Injections, Preparation of Acute Brain Slices, and Visualization of Presynaptic Fibers. (A, B) Stereotactic virus injection. A) Picture of anesthetized mouse placed in a stereotactic frame with skull exposed and the injection pipette. Inset: Zoom in picture of injection pipette filled with virus solution mixed with fast green. Scale bar: 3 mm. (B) Schematic of a mouse skull with marked positions of drill holes for different injection areas. (C) Scheme showing different viral constructs used in this study. Dark grey: promoter sequence; blue: Channelrhodopsin2 (ChR2 (H134R)); green/red: fluorescent protein. Expression time was 2 weeks for local amygdala projections and 4 – 6 weeks for projections from mPFC and MGm/PIN. (D) Preparation of acute brain slices: Scheme showing placement of mouse brain on slicer stage for obtaining slices from different injection and projection areas. (E) Visualization of fibers in acute brain slices from a GAD67-GFP mouse injected with ChR2-mCherry virus in MGm/PIN. Pictures are taken on the upright patch microscope with different filter sets: GAD67-GFP expression, middle; MGm/PIN fibers labeled with mCherry, right. Scale bar: 200 µm. mPFC, medial prefrontal cortex; mpITC, medial paracapsular intercalated cells; BLA, basolateral amygdala; MGm, medial geniculate nucleus, medial part; PIN, posterior intralaminar thalamic nucleus; LA, lateral amygdala; BA, basal amygdala; CeA, central nucleus of the amygdala.
Divulgations
The authors have nothing to disclose.
Materials
| artificial cerebrospinal fluid (ACSF) | |||
| internal patch solutions | |||
| Magnesium Sulfate Heptahydrate | Roth, Germany | P027.1 | prepare 2M stock solution in purified water |
| Slicer, Microm HM650V | Fisher Scientific, Germany | 920120 | |
| Cooling unit for tissue slicer, CU65 | Fisher Scientific, Germany | 770180 | |
| Sapphire blade | Delaware Diamond Knives | custom order, inquire with company | |
| Stereoscope, SZX2-RFA16 | Olympus, Japan | ||
| Patch microscope, BX51WI | Olympus, Japan | ||
| Bath temperature controler, TC05 | Luigs & Neumann, Germany | 200-100 500 0145 | |
| Cellulose nitrate filterpaper for interface chamber | Satorius Stedim Biotech, Germany | 13006–50 ACN |

