A Technique to Isolate Satellite Cells From Rat Head Muscle Tissue
Abstract
Source: Carvajal Monroy, P. L., et al. Isolation and Characterization of Satellite Cells from Rat Head Branchiomeric Muscles. J. Vis. Exp. (2015).
This video demonstrates the isolation of satellite cells from rat head branchiomeric muscle tissues through tissue dissection, enzymatic digestion, and subsequent mechanical and centrifugal purification. The cells are then seeded on extracellular matrix-coated wells for differentiation into myoblasts, and eventually into myotubes and mature muscle fibers.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Extracellular Matrix Gel Spots
- Perform the following steps one day before the isolation:
- Thaw an aliquot of extracellular matrix gel (100 μl) at 4 °C for at least 1.5 hr. Dilute 1:10 in Dulbecco's modified Eagle's medium; with 4,500 mg/L glucose, 4 mM L-glutamine, and 110 mg/ml sodium pyruvate (DMEM). Keep the extracellular matrix gel at 4 °C at all times.
NOTE: Abrupt temperature changes will result in uneven coating and crystal formation. - Keep the diluted extracellular matrix gel solution on ice for 15 min.
- Pre-chill a 20 μl micropipette for 10 min.
- Put 8-well chamber slides into a 100 mm Petri dish and transfer the dish onto a cold surface (e.g. a freezer pack) for 10 min.
- Use the pre-chilled micropipette to put a drop of 10 μl extracellular matrix gel in each well. Keep the Petri dish on the cold surface for at least another 7 min (Figure 1A).
- Completely remove the remaining extracellular matrix gel (Figure 1B) and dry the wells at 37 °C overnight.
- Thaw an aliquot of extracellular matrix gel (100 μl) at 4 °C for at least 1.5 hr. Dilute 1:10 in Dulbecco's modified Eagle's medium; with 4,500 mg/L glucose, 4 mM L-glutamine, and 110 mg/ml sodium pyruvate (DMEM). Keep the extracellular matrix gel at 4 °C at all times.
2. Dissection of Head Muscles (Masseter, Digastric, and Levator Veli Palatini)
- Before dissection, prepare 50 ml of phosphate-buffered saline (PBS) supplemented with 2% Penicillin-Streptomycin (P/S). Keep on ice.
- After the euthanasia of one young adult rat (9 weeks) with CO2/O2, decapitate the head and remove the skin from the head. Transfer the head to ice-cold PBS supplemented with 2% P/S in a 50 ml tube.
- Masseter muscle (derived from the 1st branchial arch)
- Place the head with one side up on a silicone pad and fix it with hypodermic needles (Figure 2A).
- Identify the parotid gland and the facial nerve (Figure 2A). Expose the deep fascia covering the gland. Cut the fascia and remove the gland using dissection scissors. Identify the external auditory canal. Trace the facial nerve from the stylomastoid foramen and carefully remove the temporal, zygomatic, and buccal branches with a scalpel blade No. 15.
- Free the superficial head of the masseter muscle by removing the fascia. Identify both superficial and deep heads of the masseter muscle. Trace the superficial head until its thick tendinous aponeurosis is inserted in the zygomatic process of the maxilla.
- Separate the tendon from its origin at the zygomatic process with straight forceps. Cut it with a scalpel blade No. 15 or dissection scissors and carefully lift it (Figure 2B).
- Dissect the superficial head of the masseter until its insertion at the angle and inferior half of the lateral surface of the ramus of the mandible with a scalpel blade No. 15 (Figure 2C). Now, completely remove the muscle.
- Posterior belly of the digastric muscle (derived from the 2nd branchial arch)
- Place the head in a supine position on the silicone pad and fix it with hypodermic needles (Figure 3A).
- Remove the subcutaneous fat overlying both sublingual and submandibular glands. Next, remove the superficial fascia and glands using dissection scissors. Expose the digastric muscle (anterior and posterior belly).
- Hold the anterior tendon of the posterior belly with straight forceps, cut it, and dissect it carefully until its origin is in the tympanic bulla (Figure 3B). Do the same on the contralateral side.
- Levator veli palatini muscle (derived from the 4th branchial arch)
- After dissection of the posterior belly of the digastric muscle, localize the stylohyoid muscle, pull it laterally, and carefully remove it (Figure 4A).
- Localize the tendon of the levator veli palatini that inserts at the tympanic bulla (Figure 4A). Dissect it carefully and cut it on both sides.
- Look for the trachea and the esophagus that runs behind it. Lift the esophagus and expose the pharynx and the larynx.
- Localize and dissect the area of the superior pharyngeal constrictor muscle. Identify the levator veli palatini and cut it at both sides (Figure 4B).
NOTE: Directly after dissection, carefully remove tendon and connective tissue from each muscle under the stereo microscope. Submerge all specimens quickly in 70% ethanol and transfer them to ice-cold PBS supplemented with 2% P/S in a 15 ml tube.
3. Isolation of Satellite Cells (SCs)
- Perform the following preparation steps for SC isolation from the three muscle groups:
- Prepare 7.5 ml of 0.1% pronase in DMEM. Filter the solution through a 0.22 μm filter. Pre-warm the solution at 37 °C in a water bath for 10 min before isolation.
- Prepare 35 ml of DMEM supplemented with 10% Horse Serum (HS) and 1% P/S. Also, pre-warm at 37 °C in a water bath.
- Prepare 15 ml culture medium which consists of DMEM supplemented with 20% fetal bovine serum (FBS), 10% HS, 1% P/S, and 1% chicken embryo extract (CEE). Pre-warm at 37 °C in a water bath.
- Pre-coat six plastic pipettes (10 ml) with HS and dry for at least 10 min before use.
- In the culture hood, transfer each muscle into a well of a 6-well plate. Using the dissection scissors, cut the muscle in small pieces of about 2 mm. Be careful not to mince the tissue too much.
- Carefully add 2.5 ml of 0.1% pronase solution to each well and incubate at 37 °C for 60 min. Gently shake the plate after 20, 40, and 60 min.
NOTE: The exact duration of the incubation depends on factors like the age and strain of the animals. - Monitor under the microscope. Check the muscle fragments and stop the enzymatic digestion when the fiber bundles appear loosened (Figure 5).
- Add 2.5 ml of DMEM supplemented with 10% HS and 1% P/S. Transfer to a 15 ml tube and centrifuge the tubes at 400 x g for 5 min. Discard the supernatant by decantation.
- Add 5 ml DMEM supplemented with 10% HS and 1% P/S. Pipette the solution up and down with a 10 ml plastic pipette (trituration) at least 20 times to homogenize the tissue.
- Centrifuge the tubes at 200 x g for 4 min. Collect the supernatant and transfer it into a 15 ml tube.
- Add 5 ml DMEM supplemented with 10% HS and 1% P/S. Pipette again with a 10 ml plastic pipette until the tissue fragments pass easily.
- Centrifuge the tubes at 200 x g for 4 min and collect the supernatant in a 15 ml tube.
- Put a cell strainer (40 μm) onto a 50 ml tube and transfer the supernatant containing the dissociated cells onto the filter. Wash with 1 ml DMEM for maximal cell recovery.
- Centrifuge the tubes at 1,000 x g for 10 min and discard the supernatant with a pipet.
- Resuspend the pellet in 300 μl culture medium and count the cells in a hemocytometer.
4. Differentiation of Satellite Cells on Extracellular Matrix Gel Spots
- Dilute the cell suspension to obtain 1.5 x 103 cells in 10 μl of culture medium.
- Secure the covers of the chamber slides with tape and mark the spots with a black marker on the bottom side of the object glass.
- Using a micropipette, put a drop of 10 μl cell suspension onto the extracellular matrix gel spot. Check under the microscope whether the drop of cell suspension has been placed correctly on the spot. Incubate for six hours at 37 °C.
- Carefully add 400 μl of culture medium (DMEM supplemented with 20% FBS, 10% HS, 1% P/S, and 1% CEE) and incubate for three days at 37 °C.
NOTE: At this point, freshly isolated SC are subjected to massive trauma (enzymatic digest and harsh trituration) and they need to recover. Do not disturb the cells during the first three days. Next, the culture medium can be changed depending on the type of experiment.
The extracellular matrix gel spots can be seeded with a high cell density (1.5-2.5 x 103/20 μl) for differentiation assay. Culture medium (DMEM supplemented with 20% FBS, 10% HS, 1% P/S, and 1% chicken embryo extract) can be replaced every third day.
Representative Results

Figure 1: Extracellular matrix gel spots in a chamber slide. (A) For easy manipulation, place the 8-well chamber slide into a 100 mm Petri dish. Pipet 10 μl extracellular matrix gel in each chamber and put it on a cold surface (7 min). (B) Chamber slide after the excess extracellular matrix gel is removed.
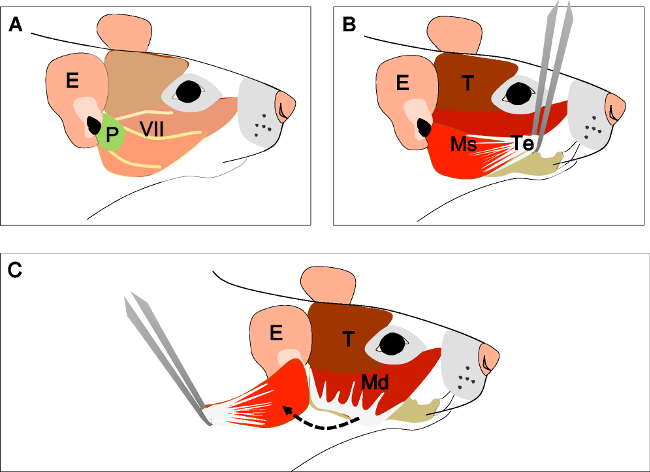
Figure 2: Dissection of the masseter muscle. (A) Head of the animal in a lateral view. Ear (E), Parotid gland (P), and facial nerve (VII). (B) Tendinous aponeurosis (Te) of the superficial head of the masseter muscle (Ms) and temporal muscle (T). Separate the tendon from its insertion with forceps. (C) Carefully dissect the muscle until its insertion at the ramus of the mandible. E: ear, P: parotid gland, VII: facial nerve, T: Temporal muscle, Ms: superficial head of the masseter muscle, Te: tendon, Mp: deep head of the masseter muscle.
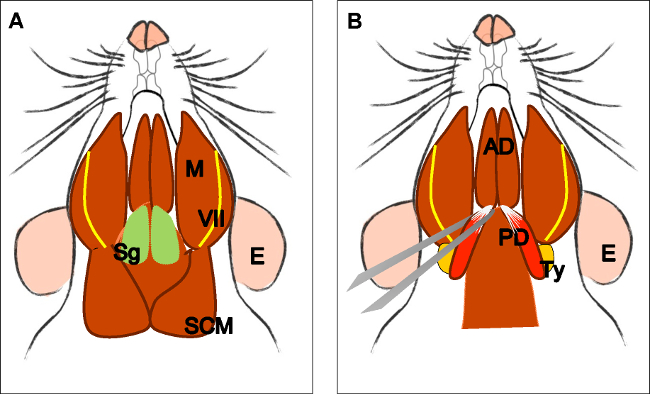
Figure 3: Dissection of the posterior belly of the digastric muscle. (A) Head of the animal in a supine position. Localize the submandibular gland (Sg), masseter muscle (M), facial nerve (VII), and sternocleidomastoid muscle (SCM). Remove the submandibular gland. (B) Localize the digastric muscle anterior (AD) and posterior belly (PD). With straight forceps, take the anterior tendon of the posterior belly, cut it, and dissect it carefully until its origin in the tympanic bulla (ty). E: ear, Sg: submandibular gland, VII: facial nerve, M: masseter muscle, SMC: sternocleidomastoid muscle, AD: anterior belly digastric muscle, PD: posterior belly digastric muscle, Ty: Tympanic bulla.
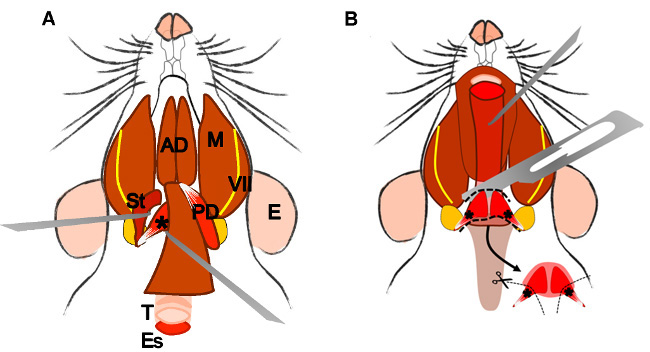
Figure 4: Dissection of the levator veli palatini muscle. (A) General view after dissection of the digastric muscle (posterior belly). Stylohyoid muscle (St) and tendon of the levator veli palatini can be localized. Note the trachea (T) and esophagus (Es) running behind it. (B) After lifting the trachea and the esophagus the pharynx (P) is exposed. The levator veli palatini that runs laterally towards the soft palate is now visible. The arrow indicates the dissected superior pharyngeal constrictor muscle; note the levator veli palatini muscles on both sides. E: ear, St: stylohyoid muscle, VII: facial nerve, M: masseter muscle, AD: anterior belly digastric muscle, PD: posterior belly digastric muscle, T: trachea, Es: esophagus, P: Pharynx, *levator veli palatini muscle.
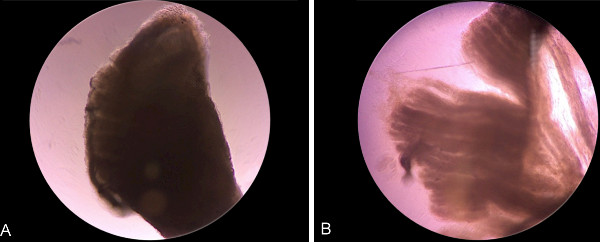
Figure 5: Appearance of the muscle tissue (A) before and (B) after enzymatic digestion with pronase. Note that muscle bundles appear to be loosened after enzymatic digestion.
Divulgations
The authors have nothing to disclose.
Materials
| Hypodermic Needle 25 G 0.5 x 25 m |
BD Microlance | 300400 | |
| Dissecting scissors | Braun | BC154R | |
| Micro forceps straight | Braun | BD330R | |
| Surgical Scalpel Blade No. 15 | Swann-Morton | 0205 | |
| Alcohol 70% | Denteck | 2,010,005 | |
| Permanox Slide, 8 Chamber | Thermo Scientific | 177445 | |
| 6 well cell culture plate | Greiner bio-one | 657160 | |
| Cell Culture Dishes (100 x 20 mm) | Greiner bio-one | 664160 | |
| 15 ml sterile conical centrifuge tube | BD Biosciences | 352097 | |
| 50 ml sterile conical centrifuge tube | BD Biosciences | 352098 | |
| Cell strainer (40 μm) | Gibco | 431750 | |
| 10 ml serological pipette | Greiner bio-one | 607180 | |
| 20 µl FT20 | Greiner bio-one | 774288 | |
| Matrigel, Phenol-Red Free | BD Biosciences | 356237 | 10 ml |
| Pronase | Calbiochem | 53702 | 10 KU |
| Phosphate Buffered Saline | Gibco | 14190-144 | 500 ml |
| Dulbecco's Modified Eagle Medium, high glucose, GlutaMAX Supplement, pyruvate | Gibco | 10569-010 | 500 ml |
| Fetal Bovine Serum | Fisher Scientific | 3600511 | 500 ml |
| Horse Serum | Gibco | 26050088 | 500 ml |
| Penicillin-Streptomycin (10,000 U/ ml) | Gibco | 15140-122 | 100 ml |
| Chicken Embryo Extract | MP Biomedicals | 2850145 | 20 ml |

