Generation of Spheroids from Human Pluripotent Stem Cells
Abstract
Source: Shaker, M. R., et al. Robust and Highly Reproducible Generation of Cortical Brain Organoids for Modelling Brain Neuronal Senescence In Vitro. J. Vis. Exp. (2022).
This video demonstrates the transformation of human pluripotent stem cells (hPSCs) into spherical brain organoids. By using specific inhibitors, the hPSCs are guided to differentiate towards a neuronal lineage, forming neuroectodermal colonies. Special growth factors are then introduced, which encourage the self-organization and proliferation of the colonies, ultimately leading to the formation of spherical brain organoids.
Protocol
1. Cortical brain organoid generation (Figure 1)
NOTE: All the steps in this section of the protocol will occur in a Class 2 biosafety hood unless stated otherwise.
- Induction of 2D neuroectodermal colonies from human pluripotent stem cells (hPSCs) 2D culture (Days -1 to 3)
- Before induction, plate the hPSC colonies on a human embryonic stem cell (hESC) qualified basement membrane matrix in a 6-well plate at 20%-30% density. Achieve this density by passaging hPSC colonies from one well of a 6-well plate at 60% confluency into three wells of a 6-well plate.
- For basement membrane matrix coating, dilute the basement membrane matrix at a ratio of 1:50 in a plain basal medium. Evenly deposit 1 mL/well of a 6-well plate, incubate for 1 hour at room temperature (RT), and then aspirate.
- Maintain hPSC colonies for 1 day in 2 mL of a serum-free cell culture medium prior to differentiation.
- On the day of hPSC differentiation, inspect the hPSC colonies using brightfield microscopy at 4x to 10x magnification to ensure healthy colonies with no detectable differentiation.
NOTE: Healthy hPSCs will form tight edge colonies with cells that have a large nucleus, very small cytoplasm, and prominent nucleoli. Differentiated iPSC colonies will exhibit clear morphological differences to that of the described hPSC colonies above, particularly around the outer edges of the colonies or at the center. - Add reagents listed in Table 1 to make up the N2 medium required for differentiation. Bring this medium to RT before use.
- Once at RT, aspirate the serum-free cell culture medium from each well of the 6-well plate and replace it with 2 mL of N2 medium gently added with a 5 mL serological pipette.
- Add the dual Suppressor of Mothers against Decapentaplegic (SMAD) inhibitors, SB-431542 (10 µM) and LDN 193189 (100 nM).
NOTE: The SMAD inhibitors can be added to the N2 medium after the medium has been placed in each well or to the required amount of N2 medium prior to replacing the serum-free cell culture medium. Inhibitors can be evenly incorporated into the medium by gently swirling the plate or inverting the tube containing the medium and inhibitors 3-4 times. - Add fresh N2 media supplemented with SB-431542 (10 µM) and LDN 193189 (100 nM) daily to each well for the next 2 days.
NOTE: Fresh N2 medium is added to reduce the prolonged exposure of cells to the Dimethyl sulfoxide (DMSO) that is used to dissolve SB-431542 and LDN 193189 compounds and prevent cytotoxicity.
- Generation of 3D neuroectodermal spheroids from induced 2D neuroectodermal colonies (Days 3 to 7)
- Lift the induced neuroectodermal colonies using dispase following steps 1.2.2-1.2.8.
- First, remove 2 mL of N2 medium from the 6-well plate, and wash 1x with HBSS to ensure that all of the N2 medium is removed.
NOTE: N2 medium can interfere with the enzyme activity of dispase, preventing adequate detachment of neuroectodermal colonies from the well. - Add 1 mL of 2.4 unit/mL dispase to each colony-containing well.
- Incubate the well for 20-25 min (maximum 30 min) at 37 °C. Check for colony detachment regularly.
NOTE: Small colonies may detach within 20 minutes. Any colonies that remain stuck down after 30 min should be ignored. - After incubation, add 1 mL of N2 medium to the well to stop the activity of the dispase enzyme and transfer the colonies into a 15 mL tube using a wide-bore P1000 pipette tip or a modified P1000 pipette tip cut with sterile scissors (making it a wide-bore P1000 tip).
- Allow the colony clumps to sink to the bottom of the tube with gravity.
NOTE: This process will take approximately 1 minute. - Once the clumps have sunk, carefully remove the supernatant with a standard P1000 pipette tip and replace it with 1 mL of fresh N2 medium. Repeat this washing step three times to ensure complete removal of dispase.
NOTE: Any remaining dispase will prevent a uniform formation of neuroectodermal spheroids and induce cell death. - After washing, resuspend the cell clumps in 3 mL of N2 medium, transfer to one well of a 6-well plate, and add 40 ng/mL of basic fibroblast growth factor (bFGF).
NOTE: If a high number of neuroectodermal colonies were detached, these colonies could be plated across two or more wells of a 6-well plate to prevent spheroids fusion. 24 h after dispase detachment of the colonies, check whether the spheroids have formed. - Maintain the spheroids in the same media for the next 3-4 days but add fresh bFGF (40 ng/mL) to each well daily to promote neuroectodermal cell proliferation, self-organizing, and induce and expand neuroepithelia.
NOTE: If spheroids have been plated at a higher density, the media is likely to turn yellow and require replacing every 2 days with fresh bFGF (40 ng/mL). However, this is not recommended, and instead, a lower number of spheroids should be maintained in each well to avoid this issue. Spheroids can be embedded in the basement membrane matrix after 3 days if neuroepithelia are evident. If neuroepithelia are not evident or do not look strong, then maintain the spheroids for another day and check again.
Table 1: N2 Medium. The table lists the reagents required to prepare the N2 medium.
| Media components | Concentration |
| DMEM Nutrient mix F12 10x 500 mL (DMEM/F-12) | |
| N2 Supplement 5 mL (100x) | Supplmented at 1% |
| B 27 Supplement 10 mL | Supplemented at 2% |
| MEM Non-Essential Amino Acids Solution (100x) | Supplemented at 1% |
| Penicillin-Streptomycin (10,000 U/mL) | Supplemented at 1% |
| 2-Mercaptoethanol 50 mL(1000x ) | Supplemented at 0.1% |
Representative Results
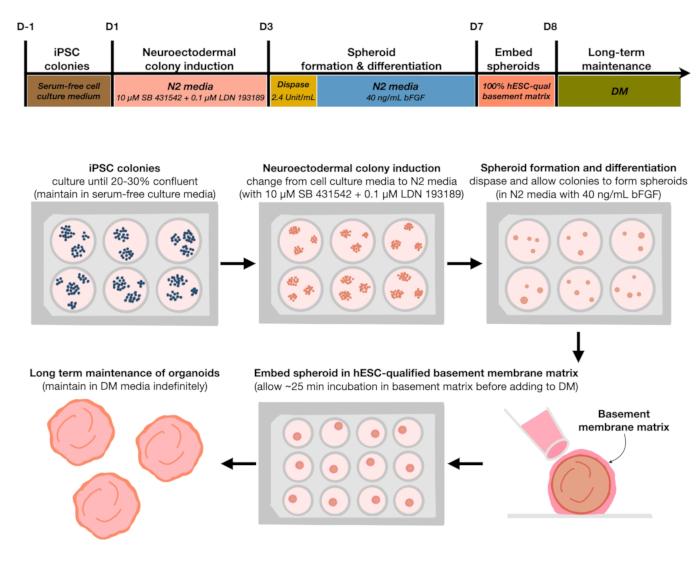
Figure 1: Schematic diagram for generating reproducible cortical brain organoids. Schematic workflow of the experimental procedure for the generation of cortical brain organoids from hPSCs maintained in the feeder-free medium. The workflow provides an overview of six steps involved in differentiating the 2D hPSCs into 3D patterned cortical plate human tissues in organoids.
Divulgations
The authors have nothing to disclose.
Materials
| 16% Formaldehyde (W/V) Methanol-free | Thermo Fisher Scientific | 28908 | 4% of PFA are diluted in 1x PBS |
| 2-Mercaptoethanol 50 mL(1000x) | Life Technologies Australia (TFS) | 21985023 | Used in NM and DM media |
| B 27 Supplement 10 mL | Life Technologies Australia (TFS) | 17504044 | Used in NM and DM media |
| CKX53 microscope with SC50 camera | Olympus | ||
| Corning Costar 6 well cell culture plates | Sigma Aldrich Pty Ltd | CLS3516-50EA | |
| Dispase II powder | Thermo Fisher Scientific | 17105041 | Powder is dissolve in HBSS, filtered through 0.22 µm filter, aliquote at 10 mL and store at -20 °C |
| DMEM Nutrient Mix F12 10x 500 mL (DMEM/F-12) | Thermofisher | 11320082 | Used in NM and DM media |
| DMSO Dimethyl Sulfoxide | Sigma Aldrich Pty Ltd | D2650-100ML | |
| Dulbecco's Phosphate Buffered Saline | Sigma Aldrich Pty Ltd | D1408-500ML | |
| Falcon Matrigel hESC-qualified Matrix | In Vitro Technologies Pty Ltd | FAL354277 | Make aliquotes of 100 µL and stored at -20 °C |
| GlutaMAX Supplement 100x | Thermo Fisher Scientific | 35050061 | Used in NM and DM media |
| Hanks Balanced Salt Solution | Sigma Aldrich Pty Ltd | H8264 | |
| Human induced pluripotent stem cells (EU79) | In-house reporogrammed from skin fibroblast | ||
| Human induced pluripotent stem cells (G22) | Genea Biocells | Obtained from Genea Biocells (San Diego, United States) | |
| Human induced pluripotent stem cells (WTC) | Gift from Professor Bruce Conklin | ||
| InSolution TGF-Β RI Kinase Inhibitor VI, SB431542 | Merck | US1616464-5MG | |
| Insulin Solution Human Recombinant | Sigma Aldrich Pty Ltd | I9278 | Used in NM and DM media |
| LDN193189 Dihydrochloride | Sigma Aldrich Pty Ltd | SML0559-5MG | Used during differentiation |
| MEM Non-Essential Amino Acids Solution (100x) | Thermo Fisher Scientific | 11140050 | Used in NM and DM media |
| mTeSR Plus | STEMCELL TECHNOLOGIES | 100-0276 | Used to maintain hiPSC colonies prior to differentiation with NM media |
| N2 Supplement 5 mL (100x) | Life Technologies Australia Pty Ltd | 17502048 | Used in NM and DM media |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | 15140122 | Used in NM and DM media |
| Recombinant Human FGF basic | R&D Systems | 233-FB-01M | Aliquots are made at 20 µg/mL and stored at -20 °C |
| SB431542 | Tocris | 1614 | Used during differentiation |
| Ultra-Low attachment multiwell plates, 24-well plate, polystyrene | Sigma Aldrich Pty Ltd | CLS3473-24EA |

