Co-culturing Glutamatergic Neurons and Pediatric High-Grade Glioma Cells
Abstract
Source: Fuchs, Q., et al. Co-culture of Glutamatergic Neurons and Pediatric High-Grade Glioma Cells Into Microfluidic Devices to Assess Electrical Interactions. J. Vis. Exp. (2021).
This video showcases a technique for co-culturing glutamatergic neurons and pediatric high-grade glioma (pHGG) cells within a microfluidic device. Human induced pluripotent stem cells are introduced into the microfluidic system and incubated to promote their differentiation into mature cortical glutamatergic neurons. Following this, the pHGG cells are introduced onto the mature neurons, establishing a co-culture and enabling interactions between neurons and pHGG cells.
Protocol
All procedures involving sample collection have been performed in accordance with the institute's IRB guidelines.
1. Cell preparation and seeding in the microfluidic device
- Culture, seeding, and immunofluorescent characterization of human hiPS-derived cortical glutamatergic neurons
NOTE: Conduct experiments with commercialized human induced pluripotent stem cells (hiPS)-derived cortical glutamatergic neurons (Figure 1A). Preserve human-derived materials and handle them with the approval and under the legislation guidelines.- Empty the inlet and outlet reservoirs of the microfluidic device by pipette aspiration before seeding, letting only the device's channels be filled with medium.
- Seed the hiPS cells at Day 0 (D0) by putting 10 µL of a 6.5 x 106 hiPS cells/mL suspension (e.g., 900 cell/mm2 concentration) with the medium, whose composition is given in Table 1, and let the device be under the hood (at room temperature) for 15 min to allow cells to attach.
- After 15 min, fill both inlet and outlet reservoirs with 50 µL of D4 culture medium, whose entire composition is given in Table 1, and transfer the device into the incubator (37 °C, 5% CO2).
- Maintain glutamatergic neuron differentiation for 23 days (Figure 1A(1), microscopy) under a controlled environment (37 °C, 5% CO2) and with a specific cell culture medium, whose composition is described in Table 1. Replace the medium regularly, as detailed in the next step 5 just below, and follow the steps in Figure 1B.
- Replace the medium every 3 to 4 days following Table 1 composition. Use seeding medium until D4, D4 medium until D7, D7 medium until D11, and D11 medium for the remaining culture time.
- Take microscopic pictures at D4, D21, and D23 using a standard phase-contrast microscope to assess cell viability and allow cell counting.
- For characterization, fix the differentiated glutamatergic neurons in 4% paraformaldehyde (PFA) for 30 min at room temperature after medium aspiration and follow the protocol for immunofluorescence staining.
- Wash cells three times with phosphate-buffered saline (PBS) and permeabilize them for 10 min with 0.1% Triton-X followed by 30 min with 3% bovine serum albumin (BSA). Add primary antibodies and incubate the device overnight at 4 °C.
- Rinse the cells thrice with PBS and incubate further with the corresponding secondary antibodies for 2 h at room temperature.
- Rinse the cells thrice with PBS and counterstain with DAPI (4',6-diamino-2-phenylindole) for 10 min at room temperature.
- Acquire images with an inverted epifluorescence microscope fitted with a CMOS (Complementary metal-oxide-semiconductor) camera and analyze using appropriate image analysis software.
- Open the DAPI stained images and binarize them with the thresholding routine in the software. To do this, click Image > Adjust > Threshold and set the appropriate parameters to distinguish the nucleus from the background. Then, click on Apply > Process > Watershed to split the aggregate nucleus.
- Then, use the Analyze Particles innate software's function to interpret the binary images. To do this, click Analyze and then Analyze Particles and set the appropriate parameters after this: size and circularity filter (exclude from analysis objects smaller than 7 µm, bigger than 20 µm, or with a circularity smaller than 0.1 µm).
- Merge the contour images obtained from DAPI analysis to correspondent immunofluorescent pictures to validate the correct staining.
- Finally, save the associated data (number of objects, mean area, % of coverage, etc.) for the different biomarkers and quantify the expression at D4 and/or D21 using appropriate quantification software.
- Cell culture of commercialized pHGG line, UW479, and patient-derived cell line, BT353
- Culture both cell lines in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) GlutaMAX supplemented with 10% Fetal Bovine Serum (FBS) in a cell culture flask. Wait for 80% confluence of each cell line, as presented in Figure 1C.
- Maintain these pHGG cells under a controlled environment at 37 °C in normoxic conditions throughout the experiments.
2. Co-culture protocol
- Adjust the seeding day and concentration for UW479 and BT35 cell lines to reach 80% of confluent cells when co-culture should start, corresponding to 21 days of culture for glutamatergic neurons.
- Trypsinize UW479 and BT35 cells and seed them on the top of glutamatergic neurons in each dedicated microfluidic device (with a target density of 900 cell/mm2 for each cell type) before seeding the pHGG cells into the microfluidic device containing matured glutamatergic neurons.
- Maintain the co-cultures for 2 days under a controlled environment (37 °C, 5% CO2) with glutamatergic neuron D11 and onward medium detailed in Table 1.
- Count pHGG cells using the microscopic pictures analyzed with an image analysis software to assess their viability. Calculate the percentage of cells.
NOTE: Use the formula: 100 – ((number of cells at D23 / number of cells at D21) x 100).
Table 1: Culture media composition for hiPS-derived glutamatergic neurons.
| Components | Seeding medium | D4 medium | D7 medium | D11 and onward medium |
| DMEM/F-12 Medium | 0.5x | 0.25x | 0.125x | Ø |
| Neurobasal Medium | 0.5x | 0.25x | 0.125x | Ø |
| BrainPhys Medium | Ø | 0.5x | 0.75x | 1x |
| SM1 Supplement | 1x | 1x | 1x | 1x |
| N2 Supplement-A | 1x | 1x | 1x | 1x |
| Ala-Gln (GlutaMax) | 0.5 mM | 0.5 mM | 0.5 mM | 0.5 mM |
| BDNF | 10 ng/mL | 10 ng/mL | 10 ng/mL | 10 ng/mL |
| GDNF | 10 ng/mL | 10 ng/mL | 10 ng/mL | 10 ng/mL |
| TGF-β1 | 1 ng/mL | 1 ng/mL | 1 ng/mL | 1 ng/mL |
| Geltrex | 30 µg/mL | Ø | Ø | Ø |
| Seeding Supplement | 1x | Ø | Ø | Ø |
| Day 4 Supplement | Ø | 1x | Ø | Ø |
Representative Results
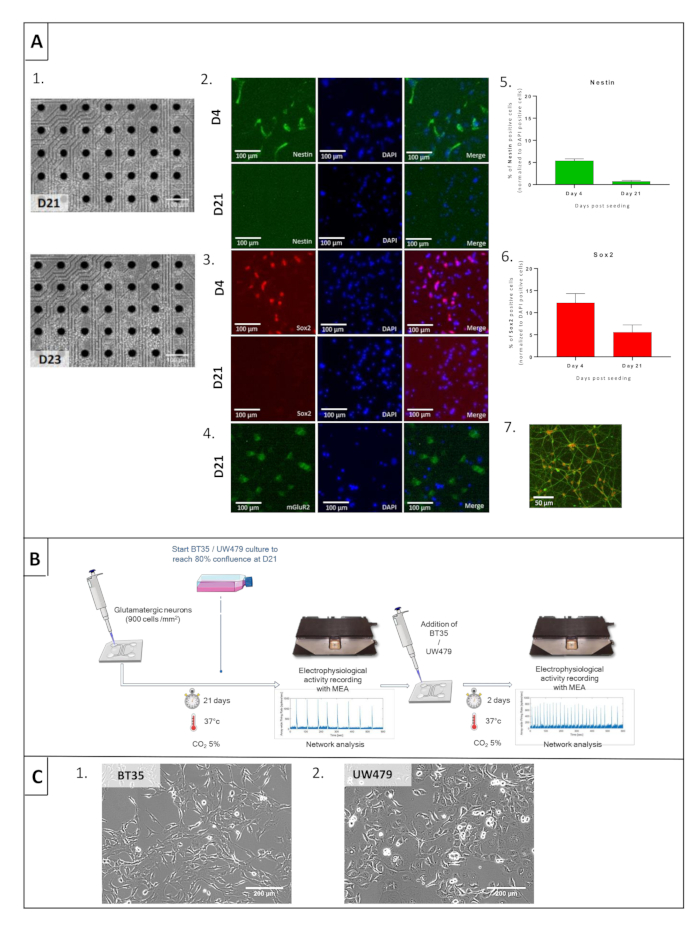
Figure 1: Cell characterization and co-culture protocol of glutamatergic neurons and high-grade pediatric glioma (pHGG) lines. (A) Prerequisite microscopic and immunofluorescent characterization of glutamatergic neurons. (1) Microscopic pictures at day 21 (D21) and day 23 (D23) of glutamatergic neurons. (2) Nestin labeling in green and DAPI in blue at D4 (upper row) and D21 (bottom row). (3) Sox2 labeling in green and DAPI in blue at D4 (upper row) and D21 (bottom row). (4) mGluR2 labeling in green and DAPI in blue at D21. (5 and 6) Statistical comparisons between Nestin and Sox2 labeling at D4 and D21 of culture, demonstrating cell differentiation. (7) vGlut1 staining in green and DAPI in orange at D21. (B) General protocol for electrophysiological recording to study interactions when co-culturing glutamatergic neurons and glioma cells on devices. (C) Prerequisite microscopic aspects of BT35 and UW479 cell lines.
Divulgations
The authors have nothing to disclose.
Materials
| 256MEA100/30iR-ITO-w/o | MCS | 256MEA100/30iR-ITO-w/o | |
| 40 µm probe for Scepter counter | Dutscher | 53750 | |
| 60 µm probe for Scepter counter | Dutscher | 51999 | |
| Accutase | Sigma | A6964 | |
| Ala -Gln (GlutaMAX) | Sigma | G8541 | |
| Axel Observer 7 Microscope | Zeiss | 431007-9904-000 | |
| Cell culture flask with cap with filter membrane 70 mL Falcon® | Dutscher | 353109 | |
| Class II Biological Safety Cabinet | Thermo Scientific | HERASafe type KS12 | |
| Colibri 7 LED | Zeiss | 4230529710-000 | |
| Cortical Glutamatergic Neurons | BrainXell | BX-0300 | |
| DMEM/F-12 (1:1) GlutaMAX | Gibco | 31331-028 | |
| DMEM/F12 Medium | Sigma | D8437 | |
| DPBS 1X | Dutscher | L0615-500 | |
| EasYFlaskTM cell culture flasks 75cm3 | Nunc | 156499 | |
| Foetal Bovine Serum (FBS) | Dutscher | 500105 | |
| GDNF | Peprotech | 450-10 | |
| Geltrex | Life Technologies | A1413201 | |
| Human BDNF | Peprotech | 450-02 | |
| Incubator | Memmert | IC0150med | |
| MCS InterFace Boarder | MCS | 181205-MEA2100-11240 | |
| MEA2100 | MCS | 181205-MEA2100-11240 | |
| Micropipette P10 | Sartorius | LH-729020 | |
| Micropipette P100 | Sartorius | LH-729050 | |
| Micropipette P1000 | Sartorius | LH-729070 | |
| Micropipette P200 | Sartorius | LH-729060 | |
| Microtube Eppendorf 1,5 ml Safe-Lock | Dutscher | 33290 | |
| MultiChannel Experimenter | MCS | – | |
| N2 Supplement-A | StemCell | 7152 | |
| Neurobasal Medium | Life Technologies | 21103049 | |
| Neurocult SM1 neuronal supplement | StemCell | 5711 | |
| Non filter tip 0.1 – 10 µl ClearLine® sterile in removable-lid rack | Dutscher | 030570ACL | |
| Non filter tip 1 – 200 µl ClearLine® sterile in removable-lid rack | Dutscher | 032260CL | |
| Non filter tip 50 – 1250 µl ClearLine® sterile in removable-lid rack | Dutscher | 134760CL | |
| Non-essential amino acids (NEAA) without L-glutamine | Dutscher | X0557-100 | |
| Pipeteur Pipet-Aid XP Gravity | Drummond | 4000202/4038202 | |
| Pipette for cell culture 10 mL Falcon® | Dutscher | 357551 | |
| Pipette for cell culture 5 mL Falcon® | Dutscher | 357543 | |
| Plaque chauffante (CultureTemp) | Belart | 370151000 | |
| Poly-D-Lysine | Sigma | P6407 | |
| Primovert microscope | Zeiss | 415510-1100-000 | |
| Scepter (Handheld Automated Cell Counter) | Millipore | PHCC00000 | |
| TGF-β1 | Peprotech | 100-21C | |
| Tube with conical bottom 15 mL (bulk) Falcon® | Dutscher | 352096 | |
| Tube with conical bottom 50 mL (bulk) Falcon® | Dutscher | 352070 |

