11.10:
Sharpless Epoxidation
3,195 Views
•
•
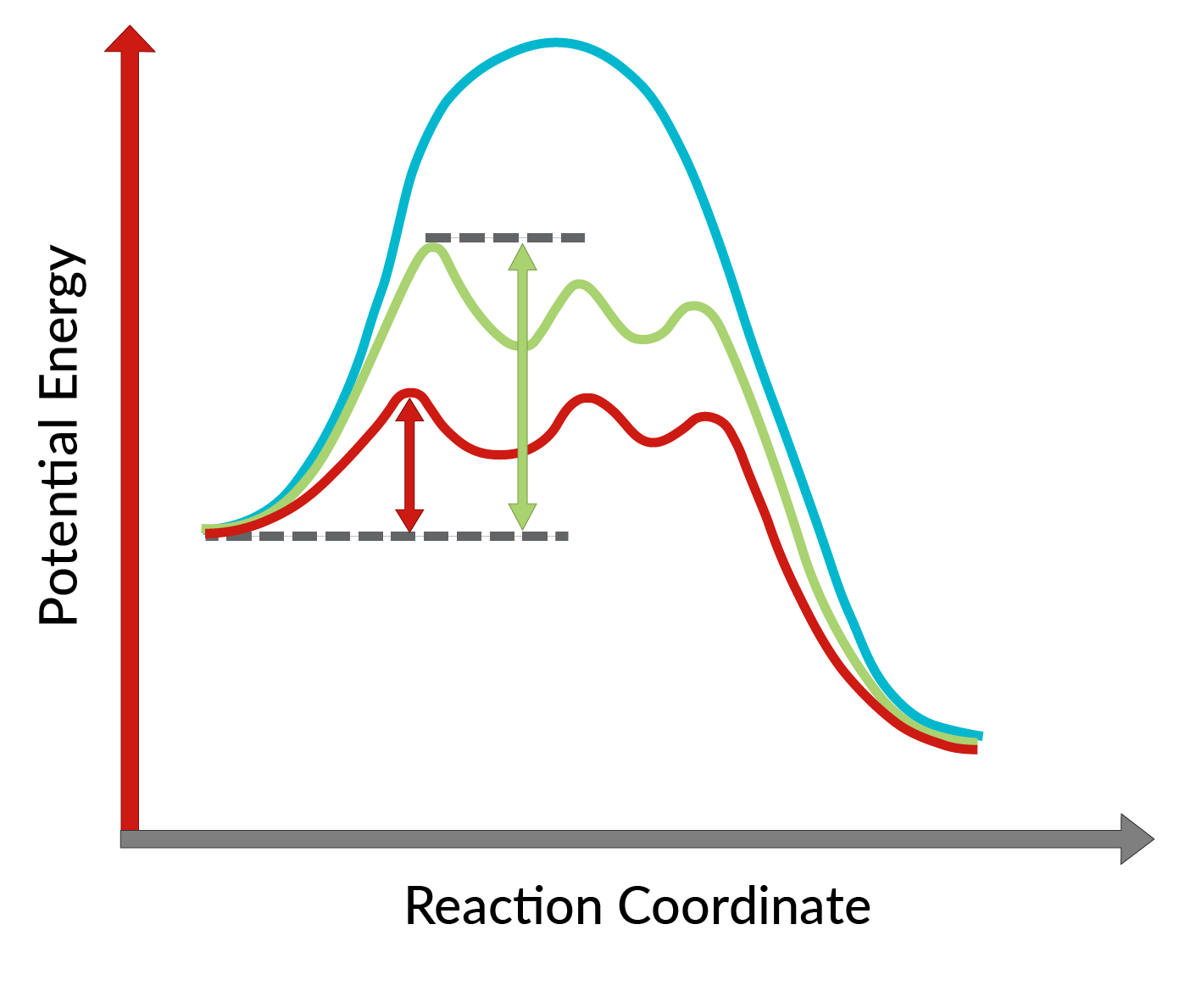
The conversion of allylic alcohols into epoxides using the chiral catalyst was discovered by K. Barry Sharpless and is known as Sharpless epoxidation. The use of a chiral catalyst enables the formation of one enantiomer of the product in excess. This chiral catalyst is mainly a chiral complex of titanium tetraisopropoxide and tartrate ester (specific stereoisomer). The stereoisomer used in the chiral catalyst dictates the formation of the enantiomer of the product. In other words, the use of L-(+)-diethyl tartrate leads to enantiomers having the epoxide ring below the plane, while with D-(−)-diethyl tartrate, to enantiomers with the epoxide ring above the plane. The high enantioselectivity of the reaction can be explained by considering the activation energies required for the reaction to proceed in the forward direction in the presence of the chiral catalyst. As shown in Figure 1, compared to the uncatalyzed reaction (blue curve), the activation energy of the reaction decreases dramatically with the addition of the chiral catalyst (red and green curves). Moreover, the activation energy for the formation of one enantiomer (red curve) is lowered more than that of another enantiomer (green curve), leading to the formation of one enantiomer in excess. Hence, Sharpless epoxidation reaction can be utilized for the synthesis of desired enantiomers of the product.
The stereochemistry of the product formed when any allylic alcohol is subjected to Sharpless epoxidation can be predicted by simply orienting the allylic alcohol molecule in a plane with the hydroxyl groups pointing towards the lower right corner, as shown in Figure 2. On this planar structure, D-(−)-diethyl tartrate delivers the oxygen from the top face of the alkene, making the epoxide formation feasible from above the plane, while L-(+)-diethyl tartrate delivers the oxygen from the bottom face of the alkene, thereby installing the epoxide ring from below the plane.
