Galvanic Cells
72,777 Views
•
•
Electrochemistry
Electrochemistry is a branch of chemistry that studies the relationship between electrical energy and a chemical change. These chemical reactions involve the movement of electrons from one species to another. This movement either generates current, or it is driven by applied current.
The key reaction in electrochemistry is the oxidation-reduction, or redox, reaction. The redox reaction is composed of two half-reactions; the oxidation reaction, where a substance loses electrons, and the reduction reaction, where a substance gains electrons. This chemical reaction results in each substance changing its oxidation state.
The atom or molecule that loses electrons, or is oxidized, is the reducing agent. The atom or molecule that accepts the electrons, or is reduced, is the oxidizing agent. One method to remember these relationships is through the phrase ‘oil-rig,’ which means, ‘oxidation is losing – reduction is gaining.’
Electrochemical Cells
An electrochemical cell is a device that generates an electric current from the energy released by a redox reaction. An electrochemical cell consists of a reaction chamber or chambers and two conductive electrodes: an anode and a cathode. The anode and cathode are connected electrically, and the reaction chamber is filled with an electrolyte. Between the two chambers is a salt bridge, which completes the circuit and allows the ions to transfer between electrodes.
Reduction occurs at the cathode, while oxidation occurs at the anode. This can be easily remembered using the mnemonic “red cat,” which means that reduction occurs at the cathode.
There are two types of electrochemical cells. One is the electrolytic cell, which uses electrical energy to drive a nonspontaneous reaction. In this type of cell, electrical energy is supplied from an external power source. The other type of cell is the galvanic cell, which uses a spontaneous reaction to generate electrical energy. Batteries are an example of galvanic cells.
Galvanic Cells
Galvanic cells, also known as voltaic cells, consist of two half-cells. Each half-cell contains a metal electrode immersed in an electrolyte. An external circuit connects the two electrodes, and a salt bridge connects the two electrolyte solutions. The electrons flow from the anode to the cathode. The oxidation half-reaction takes place at the anode, while the reduction half-reaction occurs at the cathode. For example, in a galvanic cell between copper and magnesium, the following half-reaction occurs at the cathode:
Cu2+ + 2e– → Cu
And the following half-reaction occurs at the anode:
Mg → Mg2+ + 2e–
As electrons are lost during oxidation at the anode, they travel through the external circuit to reduce the cathode, generating current.
As the anode is oxidized, the concentration of cations increases in the electrolyte. Similarly, as the cathode is reduced, the concentration of anions increases in the electrolyte. To maintain electrical neutrality, the ions travel across the salt bridge. When cations are created at the anode, anions travel from the solution on the anode side using the salt bridge. On the cathode side, anions are created, prompting cations to travel from the salt bridge to the solution on the cathode side. It is important to remember that the electrons travel through the external circuit wires, and the ions flow through the salt bridge and solutions.
Reduction Potential
Some metals have a greater tendency than others to lose electrons. Therefore, the magnitude of the electric current produced by a galvanic cell is dependent on the types of metal electrodes. The standard electrode potential (Eo) of a substance is the measure of the tendency of a substance to lose electrons. If two metals with nearly equal electrode potentials are used, then the magnitude of the current produced will be small. If two metals with very different electrode potentials are used, then the magnitude of the current will be large. The larger the reduction potential, the more likely the metal is to be reduced and act as the oxidizing agent.
Returning to the copper-magnesium galvanic cell, the standard electrode potential of copper is 0.337 volts, while the standard electrode potential of magnesium is -2.370 volts. In this example, copper is the cathode and magnesium is the anode.
When performing a galvanic cell experiment, the potential difference between the two electrodes is monitored using a multimeter. The measured voltage is equal to the difference in potential between the two half-reactions.
ΔEº = Eºcathode – Eºanode
The standard electrode potential assumes that both half-cells are under standard conditions of 1 M, 1 bar, and 298.15 K (25 °C). The voltage is dependent on the concentration of the electrolyte solutions, which can be determined using the Nernst equation.
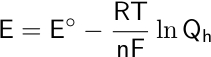
Here, E corresponds to the potential difference or the measured voltage, Eo is the standard reduction potential, R is the universal gas constant (8.314462618 J/mol·K), T is the measured temperature in Kelvin, F is Faraday’s constant (96,485.33212 C⋅mol-1), n is the number of electrons transferred in the reaction, and Qh is the reaction quotient. The reaction quotient is the electrochemical equivalent of the equilibrium constant.
References
- Kotz, J.C., Treichel Jr, P.M., Townsend, J.R. (2012). Chemistry and Chemical Reactivity. Belmont, CA: Brooks/Cole, Cengage Learning.
- Silderberg, M.S. (2009). Chemistry: The Molecular Nature of Matter and Change. Boston, MA: McGraw Hill.
- Harris, D.C. (2015). Quantitative Chemical Analysis. Sixth Edition. New York, NY: W.H. Freeman and Company.
