Isolation of Hypothalamic Microglia from Freshly Perfused Adult Mouse Brain by Magnetic-Activated Cell Sorting
Summary
We describe a protocol for isolating microglial cells from the mouse hypothalamus (or equivalent small brain structures) using magnetically activated cell sorting (MACS), in a relatively short time. The MACS-sorted hypothalamic microglia can be used for ex vivo analysis and can be plated to perform in vitro assays.
Abstract
Microglia, as the resident macrophages of the brain, are essential for maintaining brain homeostasis. They shape neuronal circuits during development, survey their environment for debris or dead cells, as well as respond to infection and injury in the brain, among many other functions. However, their important role in neurodevelopment and synaptic plasticity and pathophysiology has not been fully defined, highlighting the need for further investigation. To gain a more comprehensive understanding of the role of microglia in these processes, we need to isolate microglia and characterize them genetically, metabolically, and functionally. However, the isolation of microglia from adult mice, especially from small brain structures, is challenging as they represent a small percentage of the total brain cells, and the yield of isolated microglia is often too low. Here, the magnetic isolation of microglia using CD11b+ microbeads allows us to sort microglial cells from the hypothalamus of a freshly perfused adult mouse brain. The current method allows us to achieve relatively high purity and yield in a short period while maintaining cell viability.
Introduction
Microglia correspond to 5-20% of the total neural cells and are the only glial cells that originate from erythromyeloid progenitors in the yolk sac and start to colonize the developing brain around embryonic day E9.51,2. They are long-lived cells with the ability to undergo self-renewal slowly, independently of bone-marrow-derived cells3. As some of the most highly dynamic cells, they are capable of acquiring diverse phenotypes in response to contextual and environmental cues2,4. Among the signals able to modulate their activity, central nervous system (CNS) damage, neuronal activity, as well as nutrients, are most potent. An increasing body of research has demonstrated the pivotal role of microglia in injury, neurodegenerative diseases, and obesity2,5,6. Nevertheless, the precise role of microglia in both physiological processes and pathophysiology requires further study. Therefore, their transcriptional, metabolic, and functional characterization under different conditions is of great importance and requires their isolation, since in situ studies are appropriate for DNA, RNA, and protein localization and qualitative comparisons, as well as morphological characterization of microglia.
There are various techniques described for microglia isolation, among which are the Percoll gradient method7, flow-cytometry cell sorting (FACS)8, and magnetically activated cell sorting (MACS). The selection of the appropriate method depends on the objectives of the study and the level of purity required for downstream applications. The isolation of pure microglia from the adult mouse brain, especially from small brain structures like the hypothalamus, is challenging due to the limited number of microglial cells. The automated gentle dissociation of the hypothalamus using Miltenyi technology allows the purification of a relatively high microglia yield in a short time with the ability to proceed up to eight samples at the same time with a gentleMACS Octo Dissociator.
Tissue homogenization is followed by microglia purification using the MACS column-based technology and the supermagnetic nano-sized CD11b+ beads. Therefore, all the samples are processed exactly in the same way yielding intact CD11b+ cells. It is noteworthy that CD11b is not exclusively present in microglia but is also expressed on other myeloid lineage cells, including macrophages and monocytes9. To limit this, brain perfusion with ice-cold saline solution prior to hypothalamus extraction (see protocol step 2.6) ensures the elimination of most myeloid cells, leaving only a potentially small fraction of resident macrophages or those adhered to blood vessels. The resident macrophages and monocytes of a healthy steady state CNS constitute a minor percentage of the total brain immune cells (~10% and <2% respectively)10,11. Therefore, when brain cells are sorted with CD11b+ beads, both microglia and macrophages can be isolated, though the vast majority are microglia.
The end-yield of microglia and the maintenance of their viability let us perform ex vivo, as well as in vitro assays, with the advantage of analyzing specific brain regions of interest. The latest evidence has shown that the microglia population is highly heterogeneous, representing region-specific gene expression and morphological characteristics and function2,12,13. Therefore, the current protocol aims to isolate and analyze adult microglia in a region-specific manner. Indeed, we can characterize the genetic, transcriptional, and translational profiles of adult hypothalamic microglia using methods like RT-qPCR and RNA sequencing, as well as performing in vitro functional analysis.
Protocol
All animal experiments described were conducted in strict compliance with the European Union recommendations (2013/63/EU) and were approved by the local ethical committee of the University of Bordeaux (CEEA50) and the French Ministry of Higher Education, Research, and Innovation (non-technical summary of approved project NTS-FR-619193 v.1, 23-12-2022).
The following protocol is performed on adult C57BL/6 mice with an average age of 2-4 months. However, it could be performed in all mice ages and conditions taking into consideration that the immune cell landscape of the CNS may be altered and the percentage of the resident macrophages and monocytes may be increased (e.g. in aging and neurodegeneration).
1. Preparation of solutions
NOTE: The following volumes and solutions are strictly referred to the isolation of Cd11b+ cells.
- Determine the total volume of D-PBS/0.5% Bovine Serum Albumin (BSA) required based on the number of samples. Prepare the solution using the commercially available D-PBS with Calcium and Magnesium and BSA powder (see the Table of Materials).
- Prepare labeled dissociation tubes (C-Tubes) and fill each with 1,900 µL of buffer Z, provided in the adult brain tissue dissociation kit (see the Table of Materials). Thaw the enzymes and keep them on ice, together with all the buffers required in the following steps.
- Prepare and place the appropriate volume of 1x Phosphate-Buffered Saline (PBS) (see the Table of Materials) needed for the perfusion (step 2.6) on ice. Calculate 10 mL for each animal plus 10% in excess.
- For RT-qPCR analysis, prepare qPCR buffer per sample: 2.5 µL of thioglycerol + 250 µL of lysis buffer, using a commercially available kit to purify RNA (see the Table of Materials).
- For protein quantification, prepare lysis buffer by diluting the phosphatase inhibitor cocktail and protease inhibitor cocktail 1:100 in a mammalian extraction buffer (see the Table of Materials).
- For the detection of Reactive Oxygen Species (ROS) and phagocytosis assay, prepare 1x HBSS from the commercially available 10x HBSS (see the Table of Materials). Then, prepare FACS buffer by adding 0.2% BSA to 1x HBSS.
2. Removal of the brain
- Sedate the animal with a dose of 20 mg/kg of Xylazine; then, euthanize the mouse with an overdose of 400 mg/kg of pentobarbital and place it supine in a dissecting tray.
NOTE: Other anesthetic combinations to euthanize the animal can also work. - Fill a 10 mL syringe with 10 mL of ice-cold 1x PBS and attach it to a butterfly 21 G needle.
- Once the mouse is completely anesthetized (verify by pinching the paws to ensure a lack of response), breathing stopped, and the heart is in fibrillation, tent the skin with forceps and open the thoracic cavity with scissors, at the level of the last ribs and the sternum.
- Use the scissors to make a deep cut along the sides of the ribcage towards the top of the ribcage to expose the heart.
- Insert the 21 G needle in the distal part of the left ventricle (ensure that approximately 1/3 of the needle is inside the ventricle). Immediately make an incision through the right atrium using the scissors.
- Infuse 10 mL of ice-cold 1x PBS to clean the brain from any circulating immune cells.
NOTE: Visually check the brain to ensure that it is clean and well-perfused. - Cut the mouse head, carefully extract the brain, and place it on a Petri dish filled with ice and covered with aluminum foil.
NOTE: The Petri dish and foil help in seeing the brain pieces. It is beneficial to maintain a cool environment during the brain pieces chopping to limit metabolic activities. - Isolate the hypothalamus using forceps and cut it into small pieces with razor blades.
- To identify and dissect the hypothalamus, place the brain upside-down so that the cortex is ventral and the hypothalamus is visible dorsally on the superior surface. Separate the hypothalamus, which is located at the bottom of the brain on both sides of the third ventricle, using curved forceps. The average weight of the hypothalamus from adult C57BL/6 mice is 15 mg.
NOTE: The brain pieces must be as small as possible (<0.5 mm). Cutting into small pieces is a critical step since it allows a better dissociation and a higher yield of cells.
- To identify and dissect the hypothalamus, place the brain upside-down so that the cortex is ventral and the hypothalamus is visible dorsally on the superior surface. Separate the hypothalamus, which is located at the bottom of the brain on both sides of the third ventricle, using curved forceps. The average weight of the hypothalamus from adult C57BL/6 mice is 15 mg.
- Collect the pieces of the hypothalamus in dissociation tubes (C-Tubes) previously filled with 1,900 µL of Buffer Z (step 1.2).
NOTE: To have a good yield of microglial cells that is enough to perform assays, pool a minimum of two (for protein quantification) or three (for qPCR and RNAseq) hypothalami per tube.
3. Tissue dissociation
NOTE: All the steps must be performed on ice. Do not vortex the cell suspension in any step; only mix carefully with a 1,000 µL pipette.
- Prepare enzyme mix 2 according to Table 1 (calculating the total volume for all the tubes + 10% in excess). In each C-tube, add 50 µL of the enzyme contained in mix 1 (see Table 1) and 30 µL of enzyme mix 2. All the enzymes are provided in the adult brain tissue dissociation kit (see the Table of Materials).
- Close the C-tubes and place them upside-down in the dissociator with the heating brackets according to the manufacturer's instructions. Run the program 37C_ABDK_02.
- Prepare 15 mL snap tubes (see the Table of Materials) with 70 µm strainers on top of them. Wet the strainer with 2 mL of D-PBS.
- When the dissociation/digestion program finishes, empty the C-Tube onto the strainer and scratch with a 200 µL tip to reduce cell loss by increasing the cell filtration and reducing the adherence of tissue homogenate on the filter.
- Wash the tube with 2 x 5 mL of D-PBS, empty the washes onto the strainer, and scratch with a 200 µL tip between the washes.
- Centrifuge at 300 × g for 10 min at 4 °C and aspirate the supernatant completely. Carefully resuspend the cell pellet with the appropriate volume of cold D-PBS according to Table 1.
- Immediately mix carefully with 450 µL of density gradient reagent (Debris Removal Solution, see the Table of Materials) and overlay very gently with 2 mL of cold D-PBS (see Table 1).
NOTE: To obtain the different density gradient layers, the D-PBS must be overlaid gently drop by drop, with the least "disturbance" possible, 1 mL at a time with a 1 mL pipette. - Centrifuge at 3,000 × g for 10 min at 4 °C with slow acceleration and slow brake (if 9 is equivalent to full, use 5).
- Three phases are formed. Aspirate the two top phases completely and discard them. Make sure to not aspirate the third bottom phase.
- Fill up with 1800 µL of cold D-PBS and gently invert the tube 3 times.
- Centrifuge at 1,000 × g for 10 min at 4 °C with full acceleration and full brake and aspirate the supernatant completely. Carefully resuspend the cell pellet by pipetting slowly up and down with 2 mL of D-PBS/BSA.
4. Magnetic isolation of CD11b+ cells
- Centrifuge at 300 × g for 10 min at 4 °C and aspirate the supernatant completely. Carefully resuspend the cell pellet in 90 µL of buffer D-PBS/BSA.
- Add 10 µL of the CD11b-microbead mixture and mix well with the pipette.
- Incubate for 15 min at 2-8 °C in the fridge, not on ice; wash the cells by adding 1 mL of D-PBS/BSA and centrifuge at 300 × g for 10 min at 4 °C; aspirate the supernatant completely. Resuspend carefully in 500 µL of D-PBS/BSA.
- Launch the POSSEL2 program on the separator following the instructions on the screen. Place the small columns (see the Table of Materials) in the magnetic field.
NOTE: Either small or large columns can be chosen for magnetic cell sorting, according to the average number of cells expected. Small columns have a capacity of up to 1 × 107 labeled cells and large columns up to 1 × 108. When isolating cells from small structures, such as the hypothalamus, small columns are advised. The volumes of reagents are different when using large columns (see manufacturer's instructions). - Wet the columns (see the Table of Materials) with 500 µL of D-PBS/BSA. If the negative fraction of cells (CD11b–) is needed for further analysis, place a collection plate under the columns and then apply cell suspension in the columns.
- Perform three washing steps by adding 500 µL of D-PBS/BSA at a time in the column, each time waiting for the buffer to pass through the column.
NOTE: it is important to prevent the upper part of the column from drying out, so it is advised not to wait until all the buffer has passed through. - Transfer the total effluent of CD11b– fraction from the wells of the collection plate to 15-milliliter tubes.
- Remove the column from the separator, place it on a 5 mL tube, and pipette 1 mL of D-PBS/BSA into the column.
- Immediately flush out the magnetically labeled cell fraction by firmly applying the plunger supplied with the column.
- Eventually, count CD11b+ cells with a hemacytometer or any other method; expect ~15,000 cells per hypothalamus.
- Centrifuge the cell suspension at 300 ×g for 10 min at 4 °C and discard the supernatant.
5. Analyses
- For RT-qPCR analysis, resuspend the cell pellets with qPCR buffer (for preparation, see step 1.3) and store the tubes at – 80 °C until mRNA extraction. Isolate total RNA, then process and analyze it following the MIQE guidelines14. Finally, synthesize cDNA and perform qPCR using an RT-PCR device. See the Table of Materials for all the commercially available kits used.
- For protein quantification, wash the cell pellets with D-PBS (without BSA) at least 2x to eliminate BSA. Then, resuspend the cell pellets from two hypothalami with 20 µL of lysis buffer. Proceed with protein quantification with a protein assay kit (see the Table of Materials).
NOTE: We followed the manufacturer's instructions15 to quantify proteins to perform the serine/threonine kinases activity assay. - For the detection of Reactive Oxygen Species (ROS) in microglial cells, resuspend the pellet in 1x HBSS. Treat the cells following the manufacturer's protocol of the kit for Flow Cytometry Assay (see the Table of Materials). Then, resuspend treated cells in 200 µL of FACS buffer (for preparation, see step 1.5) and perform FACS to detect oxidatively stressed cells.
- For phagocytosis assay, incubate the sorted cells in D-PBS/BSA with fluorescent latex beads (see the Table of Materials) for 30 min. Adapt the number of beads to have 4 beads per cell on average. Then centrifuge at 300 × g for 7 min at 4 °C, resuspend in 200 µL of FACS buffer, and read the fluorescence emission by FACS (λex 575 nm; λem 610 nm).
Representative Results
The evaluation of the end yield relies on the level of purity and the quantity of isolated cells and it could be determined by RT-qPCR, cell counting, and protein quantification. The purity of the isolated magnetic cells from the hypothalamus was confirmed by the gene expression analysis of CD11b, C1qa, Gad1, and GFAP with RT-qPCR (Figure 1). CD11b and C1qa are predominantly expressed by microglia in a steady state CNS16 and Gad1 and GFAP serve as a neuronal and astrocytic marker respectively. The total cDNA from one hypothalamus ranged from 55 to 98 ng. It is noteworthy that only the positive fraction exhibited CD11b and C1qa expression, indicating the absence of any potential loss of microglial cells in the negative fraction. Primers are provided in Table 2.
Furthermore, the minor levels of the neuronal and astrocytic markers ensure that the vast majority of the isolated cells are microglia since resident macrophages and monocytes constitute only 10 % of the total immune cell landscape of a healthy brain10,11. These results prove the efficacy of the magnetic selection and pave the way for the application of RT-qPCR to assess the expression levels of all genes of interest in freshly sorted hypothalamic microglial cells. Similarly, an RNAseq analysis can be performed on hypothalamic-sorted microglia to have a comprehensive view of their transcriptome.
The quantity of the isolated cells was determined by cell counting and was found to be ~15,000 cells per hypothalamus (mentioned in protocol step 4.10). Additionally, protein concentration in sorted microglia was quantified, revealing an average concentration of 0.5 µg/µL in CD11b+ cells isolated from two pooled hypothalami (Figure 2). This knowledge allows different assays to be performed depending on a minimum protein threshold. Furthermore, by pooling more hypothalami, a higher concentration can be achieved, expanding the scope of potential analyses. The extracted proteins from hypothalamic microglia facilitate diverse techniques, including AlphaLISA immunoassays and kinase activity assays.
Moreover, the described protocol allows researchers to perform several ex vivo analyses, enabling the exploration of microglial activity and their role in the hypothalamus in both physiological and pathological contexts. The quantification of ROS production represents a crucial aspect of microglial immune response17.Therefore, after separation, microglia can be treated with commercially available reagents that allow the detection of ROS in live cells by exhibiting an increased fluorescent signal upon oxidation. Microglia magnetically isolated from three pooled hypothalami is sufficient to detect ROS by FACS, as shown in Figure 3A, though our results show a quite high variability of cell ROS production among the conditions we analyzed. It is noteworthy that, with the above-mentioned commercial kit (see the Table of Materials), we were also able to assess the viability of sorted Cd11b+ cells, thanks to a cell-impermeant dead cell stain that exhibits an increase in fluorescence upon binding DNA. Specifically, our data show that 99% of cells are alive (Figure 3B). Gating strategies were used to distinguish between alive and dead cells, as well as oxidatively stressed and non-stressed cells, based on their fluorescence properties.
Another main feature of microglial activity is phagocytosis, a dynamic and tightly regulated process that plays a key role in immune surveillance, tissue repair, and the overall homeostasis of the central nervous system18. Remarkably, Cd11b+ cells sorted with magnetic coupled beads can be cultured for 24 h and then incubated with fluorescent latex microbeads at different time points to evaluate, either by FACS or by fluorescence microscopy, alterations in the phagocytic activity under different conditions. Freshly sorted microglia can also be exposed to fluorescent beads or any type of pHrodo-coupled particles (e.g., synaptosomes) directly within the tube, and phagocytosis can be assessed by FACS analysis.
Specifically, we incubated directly in the tube containing sorted cell suspension, microglia isolated from two pooled hypothalami or a whole brain with fluorescent latex beads. Microglia that phagocyted at least one fluorescent bead can be distinguished from non-phagocytic cells by flow cytometry since phagocytic microglia will exhibit an enhanced fluorescence. Negative controls, not incubated with beads, were used for gating. In both our experimental conditions (mice fed with chow vs high-fat diet (HFD)) we observed a very low percentage of phagocytic microglia (Figure 4A). However, this result is shown here to demonstrate the efficacy of this method, which, remarkably, allows also to differentiate between cells that phagocyted 1 beads, 2 beads, 3 beads, and so on (Figure 4B). Finally, all the omics approaches could be applied to isolated hypothalamic microglia. We suggest pooling three hypothalami to have enough cells to perform these assays.
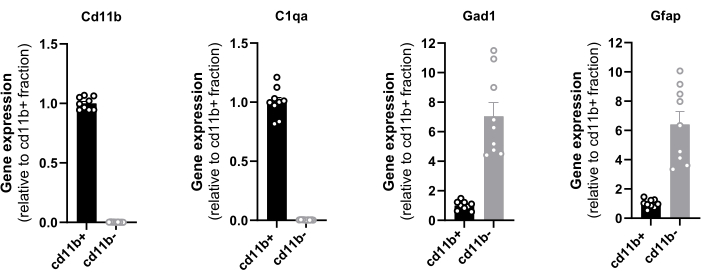
Figure 1: Evaluation of CD11b+ cell purity by RT-qPCR. Quantification in sorted cells of the mRNA expression of the integrin CD11b, complement C1q subcomponent subunit A C1q, glutamic acid decarboxylase Gad1 and glial fibrillary acidic protein GFAP normalized on mRNA levels of housekeeping genes Eef1a1 and Sdha. Please click here to view a larger version of this figure.
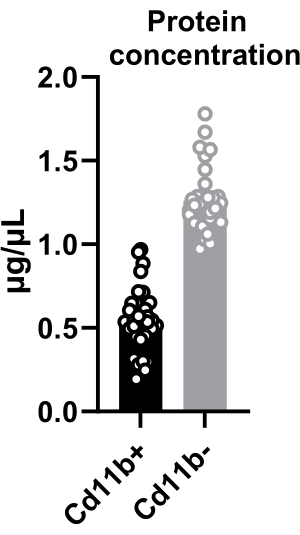
Figure 2: Protein quantification from hypothalamic cells after cell sorting. Quantification of protein concentration in CD11b+ and CD11b– cells. Both CD11b+ and CD11b– cells were lysed in 20 µL of lysis buffer. Please click here to view a larger version of this figure.
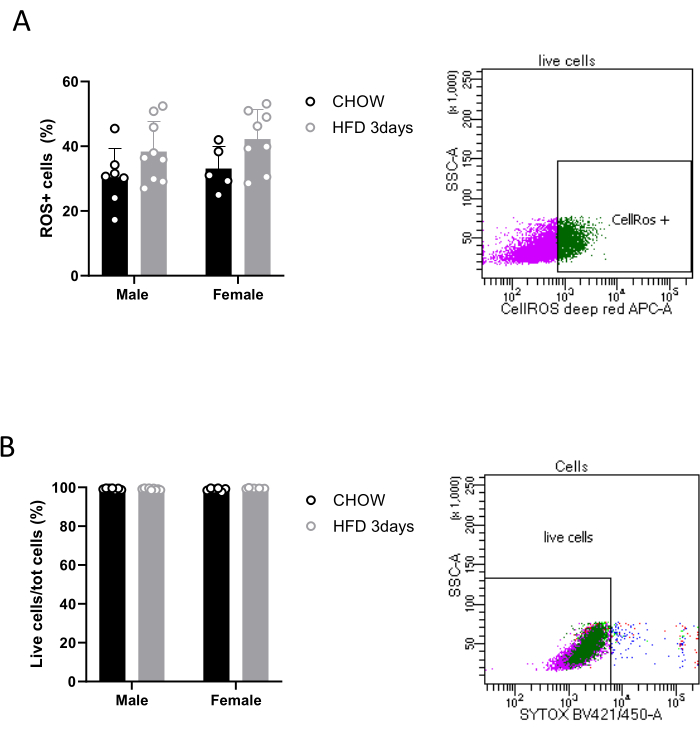
Figure 3: Detection of ROS in microglial live cells. Quantification of (A) oxidatively stressed cells and (B) live cells in a population of sorted Cd11b+ cells, with relative FACS contour plots. Please click here to view a larger version of this figure.
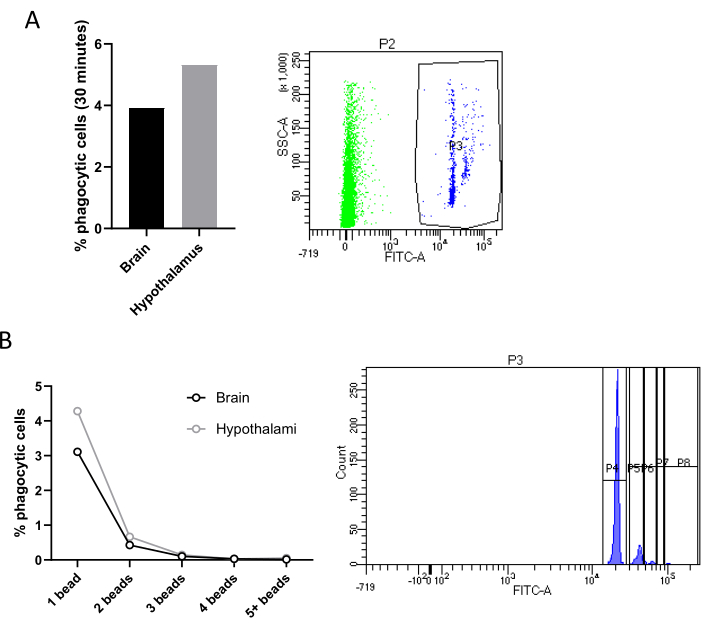
Figure 4: Quantification of microglial phagocytic activity. (A) Quantification of phagocytic cells in Cd11b+ cells sorted from two hypothalami or one brain, with relative FACS contour plot. (B) Percentage of phagocytic cells according to the number of beads phagocyted with relative FACS contour plot. Please click here to view a larger version of this figure.
| Enzyme mix 2 | |||
| Enzyme A | Buffer Y | Enzyme P | |
| 10 µL | 20 µL | 50 µL | |
| D-PBS | Debris Removal Solution | Overlay D-PBS | |
| 15-100 mg structure | 1000 µL | 450 µL | 2 mL |
Table 1: Preparation of the dissociation mixture (volumes refer to a single sample) and the gradient overlayers.
| Genes | Protein | Forward | Reverse | ||||||
| Itgam | Integrin alpha M (CD11b) | CTCATCACTGCTGGCCTATACAA | GCAGCTTCATTCATCATGTCCTT | ||||||
| Sdha | Succinate Dehydrogenase Complex Flavoprotein Subunit A | CCCTCTCATATGTGGACATCAA | CAAGGTCTTGTCGATAACAGGT | ||||||
| C1qa | Complement component 1, q subcomponent, alpha polypeptide | CGAGGTGTGGATCGAAAAGG | GAAGATGCTGTCGGCTTCAGT | ||||||
| Gad1 | glutamate decarboxylase 1 | GACCAATCTCTGTGACTCGCTTAG | CTGGTCAGTGTATCGGAGGTCTT | ||||||
| Gfap | glial fibrillary acidic protein | AGGTCCGCTTCCTGGAACA | GGGCTCCTTGGCTCGAA | ||||||
Table 2: RT-qPCR primer sequences.
| Flow-cytometry cell sorting (FACS) | Magnetically activated cell sorting (MACS) |
| Fluorescent-tagged biochemical antibodies | Magnetic beads conjugated to antibodies |
| Differentiation beyond cell surface markers | Differentiation only according to cell surface markers |
| Substantial initial yield | Modest initial yield |
| Slower than MACS for simultaneous handling of many samples | Faster than FACS for simultaneous handling of many samples |
| Less gentle than MACS | Gentler than FACS |
Table 3: Representation of FACS and MACS characteristics and limitations
Discussion
The current protocol presents the isolation of hypothalamic microglia from freshly perfused adult mouse brain by Magnetic-Activated Cell Sorting. The results presented above confirm the purity and viability of isolated cells, as well as the efficacy of this method to functionally characterize microglia ex vivo, e.g. through phagocytic activity and ROS production quantification. Classical methods for the isolation of a specific cell population could also be used for microglial cells. Density gradient fractioning is a well-known method for the separation of different cells and their particles according to their size and/or density. This technique could be applied for the isolation and culture of primary microglia. However, the purification of adult microglia requires further sorting of the isolated brain cells using fluorescent-activated or immunomagnetic cell sorting.
Specifically, FACS relies on fluorescent-tagged biochemical antibodies to label the cells and distinguish them according to a great number of variables that go beyond surface markers, such as their size and metabolic status. Nevertheless, the process of cell purification could be slower and not as gentle, increasing the number of dead cells, as well as requiring a substantial initial yield. Therefore, the small number of hypothalamic microglia is already a limiting factor. MACS utilizes magnetic beads conjugated to certain antibodies against specific cell epitopes and could work with a modest initial yield facilitating the region-specific studies on microglial dynamics. Due to the strong magnetic field of the microbeads, minimum labeling is required; hence, it is gentler than FACS, ensuring the maintenance of cell integrity.Additionally, MACS is faster than FACS when the simultaneous handling of numerous samples is necessary. This provides the distinct advantage of maintaining high cell viability that could also come from a small initial yield (Table 3).
The primary challenges in microglia isolation from the hypothalamus, which can be effectively addressed through MACS technology, include achieving a sufficient end-yield and ensuring the preservation of cell integrity. The required cell quantity depends upon the nature of the post-isolation chosen analysis. For instance, the study of the functional role of microglia in vitro requires a substantial number of cells. This underscores the importance of an initial pool of the same brain region from distinct mice with identical conditions before proceeding to the isolation process. Conversely, techniques such as high-precision single-cell sequencing do not necessitate a large cell population, and in turn, pooling several mice is not required.
To maximize cell yield, it is crucial to adhere to specific steps in the protocol. Effective dissociation is initiated by cutting the hypothalamus into small pieces, and then by ensuring that they are maintained in the buffer at the bottom of the tube during the process. Then, a combination of mechanical dissociation with enzymatic degradation of the extracellular matrix ensures a significantly high cell yield. However, we should be extremely cautious with the interpretation of the results since the enzymatic dissociation performed at 37 °C could induce transcriptional and translational changes19. To prevent technically mediated alterations, the appropriate controls with transcriptional and translational inhibitors could be used19. It is also possible to proceed to the enzymatic dissociation at a lower temperature, such as in a cold room (the effectiveness may be reduced) although dissociation may rely more on mechanical effect than enzymatic. Following dissociation, the cell suspension is prewashed and transferred to the strainers, and two more thorough washes of the tubes are performed to prevent cell loss. One more critical step is the removal of debris and myelin achieved by the density gradient centrifugation and the formation of different layers. Hence, attaining a considerable end-yield necessitates meticulous handling of both hypothalamic tissue and cell suspension, maintaining them always on ice to slow down metabolism and maintain their in vivo phenotype.
Furthermore, there are certain points that we should be extremely careful to achieve the highest purity of microglia and maintain microglia cell phenotype. Initially, perfusion performed at 4 °C, is crucial for the elimination of all circulating CD11b+ cells ensuring the highest potential microglia purity. However, in case of blood-brain barrier disruption, a higher percentage of circulating immune cells can colonize the brain interfering with the study of microglia themselves. To mitigate this issue, an alternative approach involves implementing a secondary round of magnetic isolation utilizing microbeads that are conjugated to the CD45 antibody. CD45 is a cell surface marker exhibiting high expression in macrophages20 and the subsequent flowthrough of this round comprises only the microglial cells. This strategy of using two rounds of magnetic isolation enhances the specificity of the process; nevertheless, it does take additional time, which poses a potential risk of interrupting cellular integrity and/or microglia phenotype. Hence, a crucial factor for preserving cells' functional characteristics and integrity involves swiftly isolating brain tissue and keeping it on ice until the dissociation process is initiated. In summary, this analytical approach enhances the understanding of microglial function and behavior, providing valuable insights into their dynamic responses in the hypothalamus.
Divulgations
The authors have nothing to disclose.
Acknowledgements
AN is supported by the Institut Universitaire de France (IUF), the University of Bordeaux, the French Foundation for Brain Research (FRC), the GLN (Lipid-Nutrition Group), and the National Research Agency (ANR, PRC 2023-MicroNRJ). CA was supported by the Fondation pour la Recherche Médicale (FRM-ARF201809006962). This project has received funding from the European Union's Horizon Europe Research and Innovation Program under the MSCA Doctoral Networks 2021, No. 101072759 (FuElThEbRaiNIn healtThYaging and age-related diseases, ETERNITY.
Materials
| Adult Brain Dissociation Kit | Miltenyi | 130-107-677 | The kit contains buffer Z, buffer Y, enzymes A and P, Debris Removal Solution, buffer A (to dissolve enzyme A). |
| Albumin Bovine FrV BSA | EuroMedex | 04-100-812-C | |
| CD11b (Microglia) MicroBeads, human and mouse – small size | Miltenyi | 130-093-636 | |
| CellROX Green Flow Cytometry Assay Kit | Invitrogen | C10492 | |
| Centrifuge Tube, Snap-Pop Lid 15 mL | CellTreat | 978449 | |
| DPBS, calcium, magnesium, glucose, pyruvate | Gibco | 14287-072 | |
| gentleMACS C Tubes | Miltenyi | 130-093-237 | |
| gentleMACS Octo Dissociator with Heaters | Miltenyi | 130-096-427 | |
| Halt Phosphatase Inhibitor Cocktail (100x) | Thermofisher | 78420 | |
| Halt Protease Inhibitor Cocktail, EDTA free (100x) | Thermofisher | 78437 | |
| HBSS (10x), calcium, magnesium, no phenol red | Thermofisher | 14065056 | |
| Latex beads, carboxylate-modified polystyrene, fluorescent red | Sigma-Aldrich | L3280 | |
| LightCycler 480 SYBR Green I Master | Roche | 4707516001 | This reagent was used to perform PCR. |
| MACS SmartStrainers (70 µm) | Miltenyi | 130-110-916 | |
| Micro BCA Protein Assay Kit | Thermofisher | 23235 | |
| M-PER Mammalian Extraction Buffer | Thermofisher | 78503 | |
| MS Columns | Miltenyi | 130-042-201 | Referred as small columns in the protocol. |
| MultiMACS Cell24 Separator Plus | Miltenyi | 130-098-637 | |
| PBSS, pH 7.4 | Thermofisher | 10010023 | |
| qScript XLT cDNA SuperMix | Quanta biosciences | 733-1177 | The kit was used to syntesize cDNA. |
| ReliaPrep RNA Miniprep Systems | Promega | Z6011 | The kit contains 1-Thioglycerol and BL buffer (referred as lysis buffer in the protocol) and it was used to isolate total RNA. |
| Vacutainer safety-lok 21 G | Becton Dickinson | 367282 |
References
- Ginhoux, F., Lim, S., Hoeffel, G., Low, D., Huber, T. Origin and differentiation of microglia. Front Cell Neurosci. 7, 45 (2013).
- Paolicelli, R. C., et al. Microglia states and nomenclature: A field at its crossroads. Neuron. 110 (21), 3458-3483 (2022).
- Ajami, B., Bennett, J. L., Krieger, C., Tetzlaff, W., Rossi, F. M. V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 10 (12), 1538-1543 (2007).
- Nimmerjahn, A., Kirchhoff, F., Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 308 (5726), 1314-1318 (2005).
- Alexaki, V. I. The impact of obesity on microglial function: immune, metabolic and endocrine perspectives. Cells. 10 (7), 1584 (2021).
- Gao, C., Jiang, J., Tan, Y., Chen, S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Sig Transduct Target Ther. 8 (1), 359 (2023).
- Lee, J. -. K., Tansey, M. G. Microglia isolation from adult mouse brain. Microglia: Methods Mol Biol. 1041, 17-23 (2013).
- Schwarz, J. Using fluorescence activated cell sorting to examine cell-type-specific gene expression in rat brain tissue. J Vis Exp. (2015), (2015).
- Lee, E., Eo, J. -. C., Lee, C., Yu, J. -. W. Distinct features of brain-resident macrophages: microglia and non-parenchymal brain macrophages. Mol Cells. 44 (5), 281-291 (2021).
- Korin, B., et al. High-dimensional, single-cell characterization of the brain’s immune compartment. Nat Neurosci. 20 (9), 1300-1309 (2017).
- Mrdjen, D., et al. High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 48 (2), 380-395.e6 (2018).
- Grabert, K., et al. Microglial brain region−dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 19 (3), 504-516 (2016).
- Tan, Y. -. L., Yuan, Y., Tian, L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry. 25 (2), 351-367 (2020).
- Bustin, S. A., et al. The MIQE Guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 55 (4), 611-622 (2009).
- . Available from: https://pamgene.com/wp-content/uploads/2022/03/1160-Preparation-Lysates-of-Cell-Lines-or-Purified-Cells-V4.2-2022-03.pdf (2022)
- Fonseca, M. I., et al. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflamm. 14 (1), 48 (2017).
- Simpson, D. S. A., Oliver, P. L. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants. 9 (8), 743 (2020).
- Galloway, D. A., Phillips, A. E. M., Owen, D. R. J., Moore, C. S. Phagocytosis in the brain: homeostasis and disease. Front Immunol. 10, 790 (2019).
- Marsh, S. E., et al. Dissection of artifactual and confounding glial signatures by single-cell sequencing of mouse and human brain. Nat Neurosci. 25 (3), 306-316 (2022).
- DePaula-Silva, A. B., et al. Differential transcriptional profiles identify microglial- and macrophage-specific gene markers expressed during virus-induced neuroinflammation. J Neuroinflammation. 16 (1), 152 (2019).

