Visualizing Lung Cellular Adaptations during Combined Ozone and LPS Induced Murine Acute Lung Injury
Summary
Combined ozone and bacterial endotoxin exposed mice show wide-spread cell death, including that of neutrophils. We observed cellular adaptations such as disruption of cytoskeletal lamellipodia, increased cellular expression of complex V ATP synthase subunit β and angiostatin in broncho-alveolar lavage, suppression of the lung immune response and delayed neutrophil recruitment.
Abstract
Lungs are continually faced with direct and indirect insults in the form of sterile (particles or reactive toxins) and infectious (bacterial, viral or fungal) inflammatory conditions. An overwhelming host response may result in compromised respiration and acute lung injury, which is characterized by lung neutrophil recruitment as a result of the patho-logical host immune, coagulative and tissue remodeling response. Sensitive microscopic methods to visualize and quantify murine lung cellular adaptations, in response to low-dose (0.05 ppm) ozone, a potent environmental pollutant in combination with bacterial lipopolysaccharide, a TLR4 agonist, are crucial in order to understand the host inflammatory and repair mechanisms. We describe a comprehensive fluorescent microscopic analysis of various lung and systemic body compartments, namely the broncho-alveolar lavage fluid, lung vascular perfusate, left lung cryosections, and sternal bone marrow perfusate. We show damage of alveolar macrophages, neutrophils, lung parenchymal tissue, as well as bone marrow cells in correlation with a delayed (up to 36-72 h) immune response that is marked by discrete chemokine gradients in the analyzed compartments. In addition, we present lung extracellular matrix and cellular cytoskeletal interactions (actin, tubulin), mitochondrial and reactive oxygen species, anti-coagulative plasminogen, its anti-angiogenic peptide fragment angiostatin, the mitochondrial ATP synthase complex V subunits, α and β. These surrogate markers, when supplemented with adequate in vitro cell-based assays and in vivo animal imaging techniques such as intravital microscopy, can provide vital information towards understanding the lung response to novel immunomodulatory agents.
Introduction
Acute lung injury (ALI) is a crucial pathologic response of lungs to infectious or other harmful stimuli which is marked by simultaneous activation of coagulative, fibrinolytic and innate immune systems1. Neutrophils promptly sense microbial as well as intracellular damage patterns through the Toll-like receptor (TLR) family2,3,4. Neutrophils release preformed cytokines and cytotoxic granule contents, which can then cause collateral tissue damage. The ensuing alveolar damage is marred with secondary cell death leading to release of molecules such as adenosine triphosphate (ATP)5, thus setting in a vicious cycle of immune-dysregulation.
An unsolved problem in the understanding of ALI relates to the question of how the injury is initiated within the alveolar membrane. The electron transport complex V, F1F0 ATP synthase, is a mitochondrial protein known to be expressed ubiquitously, on cell (including endothelial, leukocyte, epithelial) plasma membrane during inflammation. The cell cytoskeleton which is comprised of actin and tubulin, harbors many cell shape and function modulating as well as mitochondrial proteins, respectively. We have recently shown that blockade of the ATP synthase by an endogenous molecule, angiostatin, silences neutrophil recruitment, activation and lipopolysaccharide (LPS) induced lung inflammation6. Thus, both biochemical (ATP synthase) and immune (TLR4) mechanisms might regulate the alveolar barrier during lung inflammation.
Exposure to ozone (O3), an environmental pollutant, impairs lung function, increases susceptibility to pulmonary infections, and short low-levels of O3 exposures increase the risk of mortality in those with underlying cardiorespiratory conditions7,8,9,10,11,12,13,14. Thus, exposure to physiologically relevant concentrations of O3 provides a meaningful model of ALI to study fundamental mechanisms of inflammation7,8. Our lab has recently established a murine model of low-dose O3 induced ALI15. After performing a dose and time-response to low O3 concentrations, we observed that exposure to 0.05 ppm O3 for 2 h, induces acute lung injury that is marked by lung ATP synthase complex V subunit β (ATPβ) and angiostatin expression, similar to the LPS model. Intravital lung imaging revealed disorganization of alveolar actin microfilaments indicating lung damage, and ablation of alveolar septal reactive oxygen species (ROS) levels (indicating abrogation of baseline cell signaling) and mitochondrial membrane potential (indicating acute cell death) after 2 h exposure to 0.05 ppm O315 which correlated with a heterogeneous lung 18FDG retention16, neutrophil recruitment and cytokine release, most notably IL-16 and SDF-1α. The take-home message from our recent studies is that O3 produces exponentially high toxicity when exposed at concentrations below the allowed limits of 0.063 ppm over 8 h (per day) for human exposure. Importantly, no clear understanding exists on whether these sub-clinical O3 exposures can modulate TLR4-mediated mechanisms such as by bacterial endotoxin17. Thus, we studied a dual-hit O3 and LPS exposure model and observed the immune and non-immune cellular adaptations.
We describe a comprehensive fluorescent microscopic analysis of various lung and systemic body compartments, namely the broncho-alveolar lavage fluid (i.e., BAL) which samples the alveolar spaces, the lung vascular perfusate (i.e., LVP) that samples the pulmonary vasculature and the alveolar septal interstitium in the event of a compromised endothelial barrier, left lung cryosections, to look into resident parenchymal and adherent leukocytes left in the lavaged lung tissue, peripheral blood which represents the circulating leukocytes and the sternal and femur bone marrow perfusates that sample the proximal and distal sites of hematopoietic cell mobilization during inflammation, respectively.
Protocol
The study design was approved by the University of Saskatchewan's Animal Research Ethics Board and adhered to the Canadian Council on Animal Care guidelines for humane animal use. Six-eight week old male C57BL/6J mice were procured. NOTE: Euthanize any animals which develop severe lethargy, respiratory distress or other signs of severe distress before scheduled end point.
NOTE: Prepare the following: 27-18 G needle-blunted (will depend on the mouse tracheal diameter), appropriately sized PE tubing to fit the blunt needle (make a PE cannula for every mouse), cannula, 2 sharp scissors, 2 blunt forceps (small), 1 sharp forceps (small), 3 1 mL syringes, labelled microfuge tubes (for BAL, blood, vascular and bone marrow perfusate collection) and labelled vials (for tissue fixation), sample bags/cryovials for tissue collection, butcher's cotton thread roll (cut into adequate sized ligatures). All chemicals utilized for the experiments are indicated in the Table of Materials.
1. Ozone and LPS exposures for induction of murine lung injury
- Ozone exposure
- Prepare a custom O3 induction box. Add mouse feed, water bottle, enrichment toys and clean bedding in order and mimic the mice' housing environment.
NOTE: These steps will ensure that the mice have free access to food and water when housed in the custom induction box and will help alleviate undue stress. - Switch the O3 calibrator/generator "ON" (placed towards the inlet port) to stabilize the UV lamp before hand (around 15 min before exposures) and connect with in-line O3 monitor.
NOTE: The O3 monitor should read an average of 0.05 ppm, as sampled from the outlet at constant chamber air temperature (72 ±3 °F) and relative humidity (50±15%). - For O3 exposures, gently place mice in the custom induction box and continuously expose the mice to 0.05 ppm O3 for 2 h.
- Prepare a custom O3 induction box. Add mouse feed, water bottle, enrichment toys and clean bedding in order and mimic the mice' housing environment.
- Anesthesia and intranasal LPS administration
- Prepare 10 mg/mL stocks for ketamine and xylazine, separately.
- Prepare a cocktail by mixing 20 parts of ketamine and 1 part of xylazine. If the mouse weighs X g, inject (X/200) mL of ketamine/xylazine cocktail into the peritoneal cavity. For one mouse, prepare 1 mL of cocktail comprising of 1000 µL of the 10 mg/mL ketamine stock and 50 µL of the xylazine stock. Mix well by pipetting up and down in a microfuge tube.
NOTE: The ketamine and xylazine stocks can be prepared a week in advance. - Anesthetize the mice under light intraperitoneal (IP) ketamine (50 mg/kg)/xylazine (1 mg/kg) mix (e.g., for a 25 g mouse, inject 0.125 mL of the cocktail).
- Prepare a 1 mg/mL solution of LPS, in saline, and fill 50 µL of the solution in a pipette.
- Hold the mouse upright with its back/dorsal side on the palm, holding the ears with the same hand. Now, place 50 µL of the LPS solution18 gently outside nostrils (i.e., 25 µL on each nostril or 50 µL in one nostril; does not matter as long as the procedure is quick) and allow the mice to inhale the LPS solution.
- Instill control mice with 50 µL of sterile saline.
Caution: Do not exceed more than 100 µL for instillation, which could be fatal. Too less of a volume (i.e., <50 µL) and there is risk of only nasal deposition. - Following LPS administration, gently lay down the mice, either on their stomach or their back upon the mound of bedding with their head angled slightly downwards.
NOTE: This orientation prolongs retention of the solution in the nasal cavity19. - At 0, 2, 4, 24, 36 and 72 h after the exposure, anesthetize the mice i.p. with full dose of ketamine (200 mg/kg)/xylazine (4 mg/kg) mix (i.e., X/50 mL of the cocktail, where X is the mouse weight in grams or 0.50 mL for a 25 g mouse).
- Observe the mouse until it loses consciousness and the right reflex (i.e., does not turn back when laid in supine position). Now, check the mouse for depth of anesthesia, by monitoring the breathing (rhythmic chest excursions) and heart rate, which should fall noticeably, after 1-2 minutes.
- Next, check for pedal reflexes i.e., pinch either of the hind-limb digits and observe for retraction of the limb, as a reflex. If the mouse responds, top-up with additional 0.1 mL injections or more, as required.
NOTE: To achieve deep anesthesia, bear in mind that certain strains or obese mice usually have a larger volume of distribution and thus can be easily over-dosed, if sufficient time if not allowed for full anesthesia (which can be around 10 minutes or more). Accordingly, some strains can be resistant to anesthesia and thus require more top-ups. This knowledge is acquired after many practical observations and experience with mice of different strains and age. As a few pilot experiments were also performed immediately after O3 and LPS exposures (i.e., at the 2 h time-point), we also included some very early BAL cell analysis from those experiments to highlight the immediate effects of the combined O3and LPS exposure.
2. Sample collection
- Place the mouse on surgical tray. Now, gently put lubricating eye ointment on both the eyes to avoid fluid loss and sanitize the mouse with 70% ethanol spray.
- Make small cuts through the upper and lower skin membranes with scissors, to expose the trachea and thorax.
- Make a cut just below the sternum and expose the heart.
- Cardiac puncture
- Place a 25G needle attached to heparinized syringe into the right ventricle and draw blood by cardiac puncture.
NOTE: Blood usually draws with minimal plunger draw, if the time from exposing the heart to cardiac puncture is under a minute. After collecting around 0.4-0.5 mL, one may need to pause for a few seconds before collecting the remaining blood. This is done to let the heart pump any remaining volume into the right ventricular chamber. By the end of the blood collection, heart stops to pump. - Collect the blood and store in microfuge tubes for further processing.
- Place a 25G needle attached to heparinized syringe into the right ventricle and draw blood by cardiac puncture.
- Tracheostomy
- Carefully use tweezers/blunt forceps to clear off the tracheal region from overlying tissue. Use gloved hands more than the surgical instruments to avoid bleeding. If a lot of blood is oozing, there is risk of contaminating the BAL samples. Use cotton swabs, if necessary, although not preferred. Kimwipes are better alternatives.
- Now, cut through the ribcage to expose the lungs. Again, be cautious not to cut the sternum and the intercostal arteries or the ascending aorta; make smaller cuts while advancing through the rib cage)
- Pass a cotton ligature below the tracheal snip and leave as such for the time being.
- Snip the trachea at a suitable location in the distal 1/3rd portion, pointing towards the lungs, to allow access for a 28 G tracheal cannula.
- Insert a 28 G PE cannula (a minimal length of 3-5 mm and distal end trimmed for ease of insertion) into the trachea. Watch out for the end of the trachea before bifurcation and then pull about a mm back to avoid sampling only single lobe.
- Firmly, tie cotton ligature to hold the cannula in place. Do not collapse the cannula.
- Broncho-alveolar lavage (BAL)
- Fill 0.5 mL of PBS in a 1 mL syringe.
- Now gradually inject 0.5 mL of PBS into the cannula with the help of 1 mL syringe.
- After injecting PBS, aspirate out the syringe as long as it does not resist the suction.
NOTE: If there is resistance while aspirating the BAL fluid out, that indicates collapse of the alveolar or bronchial tissue. In that case, pull back the cannula by a miniscule to detach the cannula form the walls of the tissue. - Collect the aspirated fluid in a labelled microfuge tube and place on ice.
- Perform two more lavages in similar fashion collecting in the same vial (i.e., a total of 0.5 X 3 = 1.5 mL lavage).
- Measure the volume of collected BAL fluid. Lavage recovery should be close to 90%.
- Lung vascular perfusate (LVP)
- Cut the descending thoracic aorta at a location between the thoracic and abdominal halves, to avoid any back-up of perfusate in the lungs while perfusing through the right ventricle.
- Blot the cavity near the cut aorta end, free of blood.
- Next, perfuse the lungs with 0.5 mL of room-temp heparinized saline injected through the right ventricle.
- Collect the vascular perfusate from the cavity at the cut end of the descending thoracic aorta, in a microfuge tube, placed on ice and measure its volume.
- Ligate the right bronchus proximal to its branching from trachea (with cotton thread).
- In situ left lung fixation
- Connect a 1 mL syringe to tracheal cannula, which is long enough to insert through the previous tracheal incision.
- Draw back air in the syringe up to 0.6 mL mark.
- Then, fill the remaining syringe (up to the very end) with 2% paraformaldehyde at room temperature. Make sure that the plunger is ready to pop out without sucking back any air from the cannula.
- Now, affix the syringe with a scotch tape, to an upright container, measured up to 20 cm height.
- Gently, draw the plunger out to let the fixative flow towards the trachea. In case there are a few air bubbles in the cannula, gently place the plunger on top of the syringe and the fluid will start to flow after a few seconds.
- Let the left lung inflate to its total capacity in situ, for 5 minutes, from a 20 cm height forming a 20 cm water column.
- During this procedure, make sure to protect the right lung lobes from getting in contact with paraformaldehyde as this will affect the downstream assays.
- Place folded laboratory tissues to absorb any paraformaldehyde that might come in contact with the right lung lobes.
CAUTION: Paraformaldehyde is highly toxic. Therefore, do not inhale or place contact to any exposed parts of the body. Exercise extreme caution while handling. - During the time of paraformaldehyde instillation, sanitize the abdomen.
- Cut the right lung lobes off from the trachea making sure to remove any thread and immediately put the lobes in a labelled cryovial and drop it in liquid nitrogen for downstream molecular/biochemical cytokine analysis/RT-PCR/western blot analysis of lung homogenate.
- Carefully trim the left lung for any connective tissue or pleural membranes and dip it in 2% paraformaldehyde for 24 h at 4 °C.
- Embed the fixed lung in paraffin as per standard embedding protocol.
Caution: Do not over-heat the lung samples. - Cut the abdominal part and de-skin it from the pelvic (hip) bone.
- Separate out the left and right femur bones from the pelvic portion, in a saline filled petri dish kept on ice.
- Sternal and femur bone marrow aspirate collection
- Clean the tissue and muscles off the bones by using laboratory tissues.
- Collect the ventral ribcage and sternum into a saline filled Petri dish kept on ice.
- Cut the distal and proximal tips of the sternal and femur bones.
- Perfuse the bones 4 times with 0.5 mL of saline, using well-fitted needles (24 to 28 G) on to 1 mL syringes, and collect the fractions from each bone into labelled tubes fitted with filters and placed on ice.
NOTE: The needle gauge varies between animal size. After flushing, the femur bone should show up transparent. - Once the samples have been collected, bag the mouse in a plastic bag and put in an animal carcass freezer for proper disposal, as per guidelines of the institutional animal facility.
3. Sample processing
- Total (TLC) and differential (DLC) leukocyte counts
- Centrifuge peripheral blood, BAL, lung vascular perfusate and bone marrow (sternal and femur) samples for 10 min at 500 g.
- Collect the supernatants, flash freeze them and store them at -80 °C until further analysis.
- Reconstitute the cells in a minimum of 200 µL of PBS.
- Perform TLC by counting BAL, blood, lung vascular perfusate and bone marrow cells on a hemocytometer.
- Stain another 9 µL aliquot of BAL with a 1 µL mix of calcein green and red ethidium homodimer-1 to quantify live (green) cells due to intracellular esterase activity and any damaged BAL cells (in red) due to loss of plasma membrane integrity.
- Add 2% acetic acid to lyse RBCs, in a 1:10 ratio for blood TLC and 1:2 ratio for lung vascularperfusate TLC.
CAUTION: Adjust the cell concentrations by diluting in PBS if the concentration is more than 1×106 cells per mL (very important for the bone marrow samples). - Centrifuge the cells to prepare cytospins on slides and stain as explained below for DLCs. Count a minimum of 100 cells for differential leukocyte cell counts (DLCs).
NOTE: Typically, one can prepare two cytospins on one slide. And after 10-15 minutes of air drying, proceed with staining step. - Split the collected BAL from three pilot mice per group into two cytospins each and stain for actin/tubulin and mitochondria with active oxidative phosphorylation (reduced mitotracker). Utilize the BAL from three more mice to split into two cytospins each, for NK1.1/Gr1/CX3CR1 and ATPβ/Ki-67/CD61/Angiostatin stained slides. Split the BAL from three more mice into two cytospins each to stain for ATPα/Ly6G and CX3CR1/Siglec-F.
- Bronchoalveolar lavage (BAL) and Lung Vascular Perfusate (LVP) total protein quantification
- In order to quantify O3 and LPS induced perturbations of the vascular barrier or the relative oncotic pressure in the two pulmonary compartments (i.e., the alveolar septal (interstitial) and lung vascular perfusate (vascular) compartments), measure total protein content in the collected fluids.
- Analyze the thawed supernatant fractions for their total protein concentration using a standard detergent resistant colorimetric assay.
- Bronchoalveolar lavage (BAL) and lung vascular perfusate (LVP) chemokine analysis
- Next, analyze the chemokines in BAL and lung vascular perfusate (LVP) supernatants using a 33-plex magnetic bead-based immunoassay. This will inform about the directionality of airway/interstitium vs vascular chemokine gradients established after combined exposures.
- Analyze the following panel of chemokines : CXCL13 (B-lymphocyte chemoattractant), CCL27 (IL-11 R-alpha-locus chemokine (ILC)), CXCL5 (epithelial-derived neutrophil-activating peptide 78 (ENA-78)), CCL-11 (eotaxin-1), eotaxin-2 (CCL-24), CX3CL1 (fractalkine), GM-CSF (CSF-2), CCL1, IFNγ (interferon gamma), IL-10 (interleukin-10), IL-16 (interleukin-16), IL-1β (interleukin-1 beta), IL-2 (interleukin-2), IL-4 (interleukin-4), IL-6 (interleukin-6), CXCL-10 (interferon gamma-induced protein 10 (IP-10)), CXCL11 (Interferon-gamma-inducible protein 9 (IP-9)), KC (keratinocyte chemoattractant), MCP-1 (monocyte chemoattractant protein-1), MCP-3 (monocyte chemoattractant protein-3), MCP-5 (monocyte chemoattractant protein-5), MDC (macrophage-derived chemokine (CCL22)), MIP-1α (macrophage inflammatory protein-1 alpha), MIP-1β (macrophage inflammatory protein-1 beta), MIP-2 (macrophage inflammatory protein-2), MIP-3α (macrophage inflammatory protein-3 alpha), MIP-3β (macrophage inflammatory protein-3 beta), RANTES (regulated on activation, normal T cell expressed and secreted (CCL5)), CXCL-16, CXCL-12/SDF-1alpha (stromal cell-derived factor 1), TARC (thymus and activation regulated chemokine (TARC)), TECK (Thymus-Expressed Chemokine (CCL25)) and TNFα (tumor necrosis factor alpha).
4. Cytospin staining and lung histology
- Cytospin biochemical staining
- Encircle the cytospins with a hydrophobic pen to contain the chemicals for incubation during the procedure.
- Rehydrate the cytospins in PBS for 5 minutes.
- Fix the cytospin samples in 2% paraformaldehyde for 10 minutes, wash three times with PBS for 5 minutes each.
- Permeabilize with ice cold 70% acetone for 7 minutes and again wash three times with PBS for 5 minutes each.
- Stain for actin and tubulin or reduced Mitotracker (incubate with a mix of 2 µg/50 µL of Alexa 488 conjugated phalloidin shown in green, and 2 µg/µL of Alexa 555 conjugated mouse anti-α tubulin or reduced Mitotracker shown in red, respectively) for 15 minutes.
NOTE: Use mouse IgG1 isotype control antibody, in a separate cytospin, to validate the tubulin staining protocol. Perform same steps as explained from 4.1.1 to 4.1.8, but for isotype controls. - Remove the stains by gently tipping the mix from the slide. Incubate the slides with DAPI (4′,6-diamidino-2-phenylindole) for 5-10 min to stain nuclei.
- Wash the cytospins 3 times with PBS for 5 min each, coverslip with anti-fade mounting media and store overnight in dark at room temperature before imaging.
- Acquire images under a wide-field upright microscope equipped with a scientific camera. Ensure consistent camera exposure times set for the different fluorescent channels when imaging slides.
- Cytospin immune-fluorescent staining:
- Encircle the cytospins with a hydrophobic pen to contain the chemicals for incubation during the procedure. Rehydrate the cytospins in PBS for 5 minutes.
- Fix the cytospin samples by incubating in 2% paraformaldehyde for 10 minutes, wash three times with PBS for 5 min each.
- Permeabilize with 0.1% cold triton X-100 for 2 minutes, wash three times with PBS for 5 min each.
- Next, Fc block for 15 minutes by incubating the cytospin with 1:50 dilution of the Fc block antibody stock (to ensure that the primary mouse antibody does not cross-react non-specifically with the mouse Fc antibodies).
- Tip the slides to remove the Fc block and change to 1% BSA for and incubate the cytospin with a mix of 3 primary antibodies of interest (refer to Table 1 for the various combinations) for 30 minutes.
NOTE: Make sure to run isotype control antibodies (incubate with a mix mouse IgG1 and rat IgG2b kappa primary antibodies by performing steps 4.2.1 to 4.2.9 and replacing the antibodies with isotype controls) in parallel with corresponding secondary antibodies as discussed in our recent study20. - Wash 3 times with PBS for 5 min each. Incubate the cytospins with a mix of appropriately designed secondary antibodies (please refer to Table 1 for the details).
- Remove the stains by gently tipping the mix from the slide, incubate with DAPI (4′,6-diamidino-2-phenylindole) for 5-10 min to stain nuclei. Wash 3 times with PBS for 5 min each.
- Coverslip with anti-fade mounting media and store overnight in dark at room temperature before imaging.
- Acquire images under a wide-field upright microscope equipped with a scientific camera. Ensure consistent camera exposure times set for all the fluorophore channels when imaging slides.
- Hematoxylin and eosin (H&E) histology
- Perform modified H&E staining3 on lung cryo-sections for all groups.
- Image analysis
- Process and analyze image data (.tiff) files in Fiji ImageJ open software (https://imagej.net/Fiji/Downloads).
- Check the required parameters (Area, Perimeter, Integrated Density, Shape descriptors, Feret's diameter, Circularity, Display label) to be recorded under the Analyze tab and Set Measurements.
- Using the ROI manager, manually outline around 50-200 cells in the merged image panel using the "freehand selections" tool. Save the regions of interest (ROI) with Ctrl+T command and copy the saved ROIs for each cell over to all the fluorophore channels.
- Next press Ctrl+M to measure the pre-set parametersfor all the fluorescent channels (e.g., 405 nm (DAPI or ATPβ in blue), 488 nm (actin or NK1.1 or Ki-67 in green), 568 nm (tubulin or Gr1 or CD61 in red) and 633 nm (CX3CR1 or angiostatin in magenta)).
- Save the Results as .csv file under an appropriate name that represents your analysis. Copy the Results from the .csv file over to a spreadsheet and divide the fluorescence intensity (FI) (which is the Raw Integrated Density column from the Results file) of the stained molecule by that of DAPI or CD61 FI, as per staining design. These ratios are termed as "DAPI or CD61 normalized FI ratios".
- Next, plot these normalized ratios, circularity, cell perimeter and Feret diameter, to evaluate any change in cell size after the combined O3 and LPS exposure.
- Use appropriate statistical software to check the normality of the collected data and to test the null hypothesis (please refer to the statistical analysis section below).
- Statistical Analysis
- Express results as mean ± SEM. A minimum of three mice were used per group.
- For chemokine data analysis, adjust one-way ANOVA p-values for false discovery rate according to the Benjamini and Hoshberg correction.
- Analyze image parameters, cellularity, Feret diameter, perimeter, DAPI or CD61 normalized fluorescence intensity ratios by Mann-Whitney U test (for comparing two groups) or Kruskal Wallis test (for comparing multiple groups) as these data were not normally distributed.
- For rest of the imaging experiments, analyze the results using a one-way analysis of variance followed by Sidak's multiple comparisons. p-values <0.05 were considered significant.
Representative Results
Combined O3 and LPS exposure leads to systemic inflammation and bone marrow mobilization at 72 h: Cell counts in different compartments revealed significant changes in peripheral blood and the femur bone marrow total cell counts upon combined O3 and LPS exposures. Although combined O3 and LPS exposures did not induce any changes in the total BAL (Figure 1A) or LVP (Figure 1B) cell counts, polymorphonuclear cells presented as the predominant cell type at 24 (Figure 1F, Supplemental Figure 1), 36 and 72 h after exposure. Please note the absence of Siglec-f and CX3CR1 staining in polymorphonuclear cells at 24 h BAL cytospin image (Figure 1F). Triple DAPI/CD11b/Gr1 staining of BAL cytospins showed presence of large CD11b- and Gr1-positive mononuclear cells which are macrophages at 0 h, which changes to smaller polymorphonuclear cells showing punctate Gr1staining but devoid of CD11b staining at 4 h (as shown in the inset in Supplemental 1F). The majority of BAL cells are polymorphonuclear and positive for both CD11b and Gr1, at 24 h post exposure (Supplemental Figure 1).
The mice displayed systemic leukocytosis at 24 h (3.3-fold vs 0 h, p<0.05, Figure 1C) followed by leukopenia at 72 h which was marked by13-fold lower counts in peripheral blood compared to 24 h (p<0.01) and a 8.6-fold lower compared to 4 h (p<0.05) after combined O3 and LPS exposure (Figure 1C). The sternal bone marrow cells were also unchanged when compared to 0 h or after combined O3 and LPS exposure (Figure 1D). The femur bone marrow counts showed a late 28.8-fold spike at 72 h when compared to 0 h (p<0.01, Figure 1E) as well as other time points (p<0.01, Figure 1E). Visualization of the LVP, and sternal as well as femur bone marrow cells also showed changes in the cell types to predominantly polymorphonuclear cells (Supplemental Figure 1). The sternal bone marrow cells showed signs of damage as evidenced by extracellular nuclear material. The femur bone marrow showed higher numbers of CD11b and Gr1 positive cells (Supplemental Figure 1), which coincide with the cell counts.
BAL total protein content was highest at 36 h after combined O3 and LPS exposure: Quantification of the total proteins in the LVP and BAL compartments was carried out to correlate ozone induced lung edema i.e., plasma protein exudation due to resulting vascular and epithelial barrier impairment due to the combined exposure. BAL total protein was 4.5-fold higher at 36 h (p<0.05) when compared to 4 h (Figure 2A). However, LVP protein was unchanged after exposure (Figure 2B).
Combined O3 and LPS exposure induces strong chemokine gradients in the lung vascular compartment and not BAL: Chemokine multiplex assays were conducted on both BAL and LVP supernatants. Please note that the relative differences between these two compartments represents preferential retention in a particular compartment. The eosinophil chemoattractant eotaxin-2 was 4.3-fold higher at 4 h in the LVP compartment compared to 0 h (p<0.05, Figure 3A). In the BAL compartment, eotaxin-2 was 3.2-fold lower at 4 h when compared to 0 h (p<0.01, Figure 3B). The BAL eotaxin-2 levels kept on decreasing consistently until 72 h with 12.6-fold reduction compared to 0 h (p<0.01, Figure 3B). The lymphocyte chemoattractant IL-2 was 10.1-fold higher at 4 h in the LVP compartment compared to 0 h (p<0.05, Figure 3C). In the BAL compartment, IL-2 was 5.0-fold lower at 36 h when compared to 0 h (p<0.05, Figure 3D). The BAL eotaxin-2 levels kept on decreasing consistently 5.9-fold reduction at 72 h compared to 0 h (p<0.05, Figure 3D).
Interestingly, many chemokines were altered at 4 h, in the LVP and not the BAL. Most of these chemokines showed modest, yet significant, increase in LVP levels at 4 h when compared to later time-points. Although eotaxin-1 levels were not high after the exposures when compared to 0 h, but the levels were significantly high at 4 h when compared to 24 (2.7-fold, p<0.05) and 72 h (5.0-fold, p<0.01, Figure 4A) after combined exposure. The levels of LVP TNFα were 3.4-fold higher at 4 h when compared to 36 h (p<0.05, Figure 4B). Similarly, LVP IFNγ was 2.9-fold higher at 4 h when compared to 72 h (p<0.05, Figure 4C). CX3CL1 levels were also high at 4 h when compared to 24 (1.7-fold, p<0.05), 36 (1.6-fold, p<0.05) and 72 h (2.2-fold, p<0.01) after combined exposures (Figure 4D). CCL27 levels showed higher levels at 4 h as well when compared to 24 (2.7-fold, p<0.05), 36 (3.9-fold, p<0.01) and 72 h (2.9-fold, p<0.05) after combined exposures (Figure 4E).
Some chemokines had a strong presence in the LVP at 4 h after combined exposures, and not changed in the BAL before and after exposure, , indicating a strong chemokine response from the pulmonary capillaries. At 4 h, IL-16 LVP levels were 3.4-fold compared to 0 h (p<0.01), 3.2-fold compared to 24 h (p<0.01), 4.5-fold compared to 36 h (p<0.01) and 4.6-fold compared to 72 h (p<0.01) after combined exposures (Figure 4F). At 4 h, the neutrophil chemokine, CXCL5 LVP levels were 2.0-fold compared to 0 h (p<0.01), 4.7-fold compared to 24 h (p<0.01), 2.2-fold compared to 36 h (p<0.01) and 2.7-fold compared to 72 h (p<0.01) after combined exposures (Figure 4G). At 4 h, CXCL10 LVP levels were 2.3-fold compared to 0 h (p<0.01), 2.1-fold compared to 24 h (p<0.05), 2.5-fold compared to 36 h (p<0.01) and 2.7-fold compared to 72 h (p<0.01) after combined exposures (Figure 4H). At 36 h, the neutrophil chemokine, SDF1α LVP levels were 4.2-fold compared to 0 h (p<0.01), 2.9-fold compared to 24 h (p<0.05), 6.8-fold compared to 72 h (p<0.01) after combined exposures (Figure 4I). At 4 h, MIP3β LVP levels were 2.3-fold compared to 0 h (p<0.05), and 2.3-fold compared to 72 h (p<0.05) after combined exposures (Figure 4J).
Combined O3 and LPS exposure induces necrosis, disrupts cellular cytoskeletal, plasma membrane and mitochondrial integrity: As the compartmental leukocyte counts and protein contents were not correlating with the LVP chemokine gradients, it became more important to visualise the cells obtained from these compartments. Ex vivo actin/tubulin staining of baseline (0 h) BAL cells showed presence of cortical actin, stress fibers as well as lamellipodia (shown in green, Figure 5 left upper panel) and microtubule network (shown in red, Figure 5 left upper panel) that coincided with reduced mitotracker staining indicating presence of active mitochondria (shown in red, Figure 5 left lower panel). More than 95% of the BAL cells were mononuclear at baseline. At 4 h, BAL cells showed extracellular nuclear material, punctate cytoplasmic actin staining devoid of lamellipodia and reduced cortical actin staining (shown in green, Figure 5 right upper panel),fanned microtubule network (shown in red, Figure 5 right upper panel) that did not correspond to the reduced mitotracker staining (shown in red, Figure 5 left right panel). DAPI normalized actin fluorescent intensity analysis showed a 3.7-fold decrease in the ratio indicating reduction in actin staining at 4 h after exposure (p<0.01, Figure 6A). Similarly, DAPI normalized tubulin fluorescent intensity also reduced by 1.5-fold after exposure (p<0.01, Figure 6B), indicating a reduction in tubulin staining. The loss of lamellipodia was evident with an increase in the circularity of BAL cellular cytoskeleton immediately i.e., at 2 h (p<0.01, Figure 6C) and 4 h (p<0.05, Figure 6C) after exposure when compared to 0 h and a decrease in the cytoskeletal circularity at 24 (p<0.05) and 36 h (p<0.01) after exposure, when compared to 0 h (Figure 6C).
Up to 24 h, the BAL cells from the combined exposure were double positive for calcein (in green, Figure 7) indicating presence of active esterase activity, and ethidium homodimer (in red, Figure 7), indicating that although some cells were dead (red), majority were partially compromised cells. At 36 h, the BAL cells were largely viable (green), indicating replacement of the compromised cells by leukocytes recruited from other systemic compartments (Figure 7).
Specific ATPα and Ly6G staining of the BAL cells revealed discrete intracellular localization (Movies 1 and 2) of both the proteins in alveolar macrophages as well as neutrophils after exposure (Figure 7). The combined exposure led to reduction in the DAPI normalized fluorescent intensity ratio of ATPα (Figure 7, 8A) and an increase in intracellular Ly6G protein content (Figure 7, Figure 8B, Movies 1 and 2). Reduction in ATPα staining, at 24 h after exposure, correlated with lower tubulin and to some extent reduced mitotracker staining. We also observed anuclear ATPα positive cell bodies after exposure, which could be platelets. However, we did not confirm our findings. At 2 h, we observed smaller mononuclear BAL cells (p<0.01 Figure 8C, p<0.01 Figure 8D) when compared to 0 h but at 4 h, the mononuclear cells were larger (p<0.01 Figure 8C, p<0.01 Figure 8D) when compared to 0 h. At 24 (p<0.01 Figure 8C, p<0.01 Figure 8D) and 36 h (p<0.01 Figure 8C, p<0.01 Figure 8D), the BAL cells became progressively smaller owing to a shift towards polymorphonuclear cells, when compared to 0 h.
Immunophenotyping of BAL cells revealed transient expressions of NK1.1, ATP β and Ki-67 and sustained expressions of Gr1, CX3CR1 and angiostatin positive BAL cells after combined O3 and LPS exposures: Immunofluorescent staining of the first set of BAL cytospins were normalized to DAPI staining. There was an increase in the cellular NK1.1 positive cells at 24 h (p<0.05 vs 0 h, Figure 9, 10A). At 36 h, the BAL cells had lower cellular NK1.1 fluorescent intensity (p<0.01 vs 0 and 24 h, Figure 9, 10A). The cellular Gr1 fluorescent intensity was higher at 24 h (p<0.01 vs 0 h, Figure 9, 10B) as well as 36 h (p<0.01 vs 0 h, Figure 9, 10B). Similarly, the cellular CX3CR1 fluorescent intensity was higher at 24 h (p<0.01 vs 0 h, Figure 9, 10C) as well as 36 h (p<0.01 vs 0 h, Figure 9, 10C).
When normalized to cellular CD61, which is also ubiquitous in expression, revealed an increase in the cellular ATPβ fluorescent intensity at 24 h (p<0.01 vs 0 h, Figure 9, 10D) and a decrease in the cellular ATPβ fluorescent intensity at 36 h (p<0.01 vs 0 and 24 h, Figure 9, 10D). Notably, ATPβ showed predominantly nuclear localization at 24 h compared to peripheral localization at 36 h (Figure 9). The cellular fluorescent intensity of BAL Ki-67, an indicator of cellular proliferation, was higher at 24 h (p<0.01 vs 36 h, Figure 9, 10E) compared to 0 and 36 h. The levels were below baseline levels at 36 h (p<0.01, Figure 9, 10E), most likely due to higher polymorpho-nuclear CD61 vs Ki-67 expression at 36 h (Figure 9). Lastly, the cellular fluorescent intensity of BAL angiostatin was consistently high at both 24 and 36 h (p<0.01 vs 0 h, Figure 9, 10F), indicating a specific increase in this metalloproteinase cleaved plasminogen fragment, during the lung inflammatory response.
Combined exposures produce wide-spread lung damage: Lung histology revealed that the combined exposures produce prolonged damage to lungs as seen in H&E stained cryosections (Figure 11). Although bronchiolar and alveolar septal damage was expected, it was surprizing to observe endothelial damage of larger vessels, at 36 h after exposure (Figure 11). There were adherent intravascular leukocytes, patches of leukocyte aggregates (including neutrophils) in the damaged alveolar septal and peri-bronchiolar regions (Figure 11).
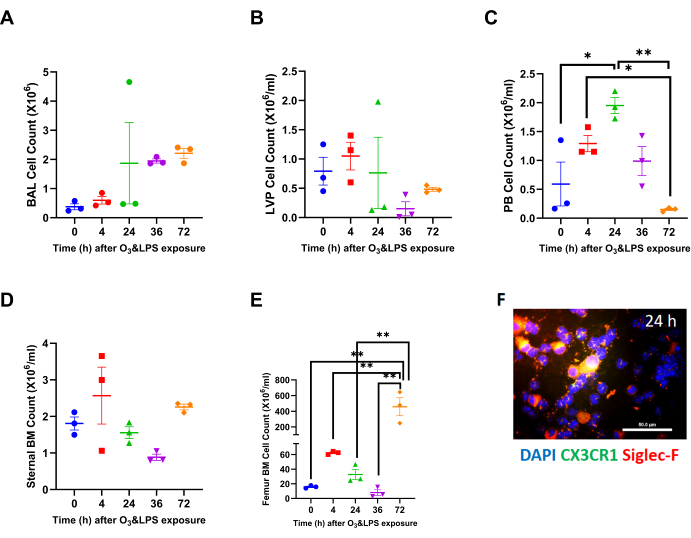
Figure 1: Compartmental leukocyte counts: A) Broncho-alveolar lavage (BAL) total leukocyte counts at 0 (i.e., baseline), 4, 24, 36 and 72 h after combined 0.05 ppb ozone (O3) and 50 µg intranasal LPS exposure to mice. B) Lung vascular perfusate (LVP) total leukocyte concentration at 0 (i.e., baseline), 4, 24, 36 and 72 h after combined 0.05 ppb ozone (O3) and 50 µg intranasal LPS exposure to mice. C) Peripheral blood (PB) total leukocyte concentration at 0 (i.e., baseline), 4, 24, 36 and 72 h after combined 0.05 ppb ozone (O3) and 50 µg intranasal LPS exposure to mice. D) Sternal bone marrow (BM) total leukocyte concentration at 0 (i.e., baseline), 4, 24, 36 and 72 h after combined 0.05 ppb ozone (O3) and 50 µg intranasal LPS exposure to mice. E) Femur bone marrow (BM) total leukocyte concentration at 0 (i.e., baseline), 4, 24, 36 and 72 h after combined 0.05 ppb ozone (O3) and 50 µg intranasal LPS exposure to mice. For the graphs A-E, 0 h is represented in blue, 4 h in red, 24 h in green, 36 h in purple and 72 h in orange data points; * p<0.05 and ** p<0.01. F) Representative BAL cytospin slide from 24 h exposure, showing mononuclear and polymorphonuclear cells when stained for nuclei with DAPI (in blue), CX3CR1 (in green) and Siglec-F (in red). Note that the merge of CX3CR1 and Siglec-F shows up as orange-yellow. Majority of mononuclear cells are positive for Siglec-F, which is a characteristic of alveolar macrophages. Scale bar = 50 µm. Please click here to view a larger version of this figure.
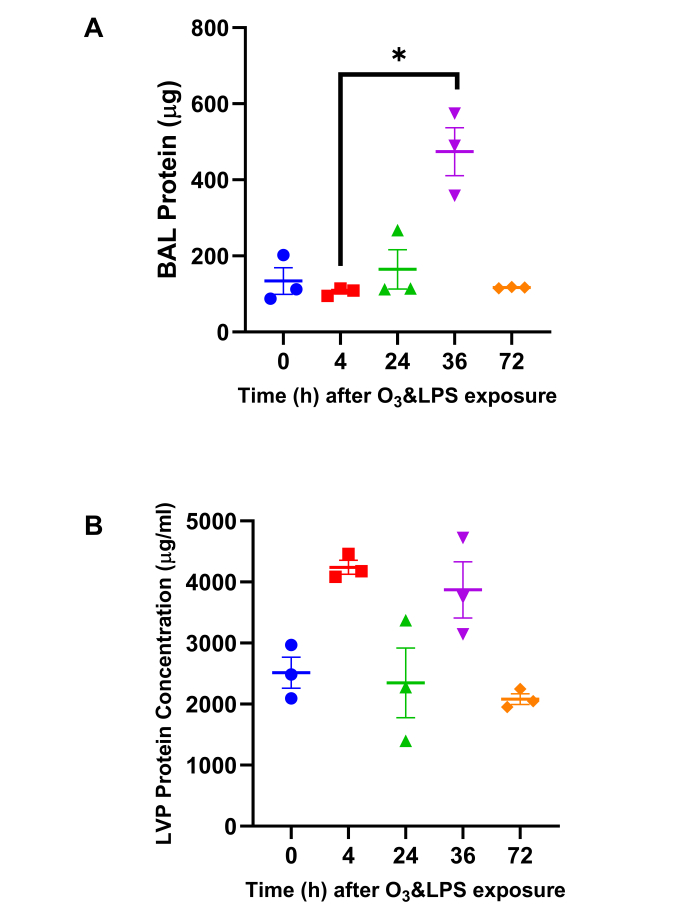
Figure 2: Total Protein quantification: A) Bronchoalveolar lavage (BAL) total protein content and B) Lung vascular perfusate (LVP) total protein concentration was estimated byPierce 660 nm protein assay (Thermoscientific, IL, US). For the graphs A-B, 0 h is represented in blue, 4 h in red, 24 h in green, 36 h in purple and 72 h in orange data points; * p<0.05. Please click here to view a larger version of this figure.
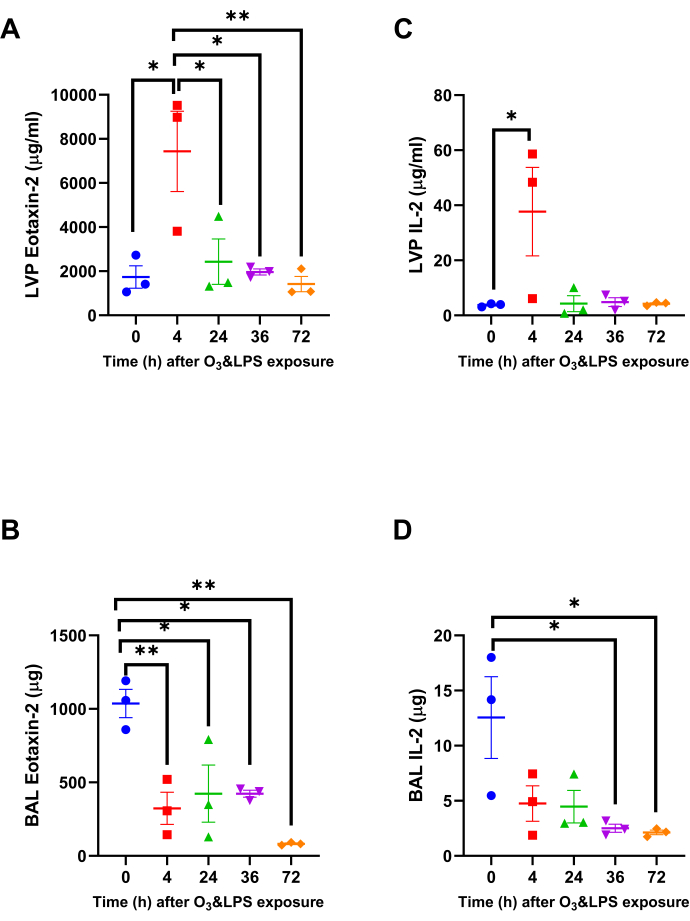
Figure 3: Lung Eotaxin-2 and IL-2 chemokine gradients: Out of the 33 chemokines, two chemokines that were significantly altered in the lung vascular perfusate (LVP) and bronchoalveolar lavage (BAL) and fluid after combined 0.05 ppb ozone (O3) and 50 µg intranasal LPS exposure to mice A) LVP eotaxin-2, B) BAL eotaxin-2, C) LVP IL-2 and D) BAL IL-2. Data were analyzed by one-way anova and the p-value for false discovery rate of multiple variables was adjusted as per Benjamini and Hoshberg correction. For the graphs A-D, 0 h is represented in blue, 4 h in red, 24 h in green, 36 h in purple and 72 h in orange data points. Pair-wise comparisons were analyzed after Bonferroni's correction. * represents p<0.05, ** represents p<0.01. Please click here to view a larger version of this figure.
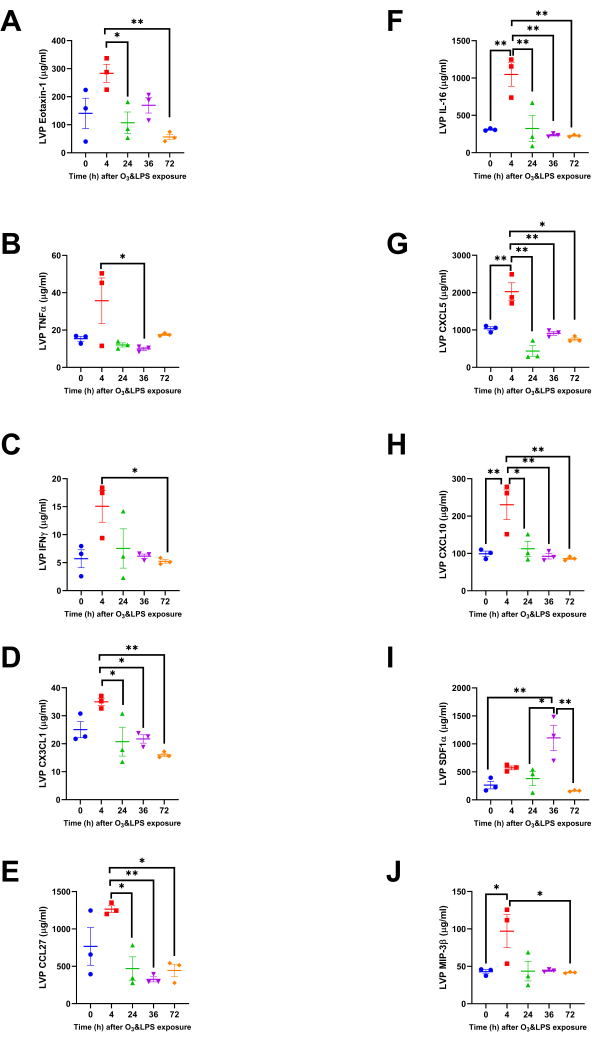
Figure 4: Lung vascular perfusate chemokine concentrations: Concentrations of ten more chemokines were altered, but only in the lung vascular perfusate (LVP) compartment. A) Eotaxin-1, B) TNFα, C) IFNγ, D) CX3CL1, E) CCL27, F) IL-16, G) CXCL5, H) CXCL10, I) SDF1α and J) MIP3β. For the graphs A-J, 0 h is represented in blue, 4 h in red, 24 h in green, 36 h in purple and 72 h in orange data points. Pair-wise comparisons were analyzed after Bonferroni's correction. * represents p<0.05, ** represents p<0.01. Please click here to view a larger version of this figure.

Figure 5: Bronchoalveolar lavage (BAL) cytospin actin/tubulin and reduced mitotracker staining: Representative images ofactin/tubulin stained BAL cytospins from A) 0 h and B) 4 h after combined 0.05 ppb ozone (O3) and 50 µg intranasal LPS exposure to mice. Note that DAPI is shown in blue, actin in green and tubulin in red. Representative images ofreduced mitotracker stained BAL cytospins from A) 0 h and B) 4 h after combined 0.05 ppb ozone (O3) and 50 µg intranasal LPS exposure to mice. Note that DAPI is shown in blue, and reduced mitotracker in red. Scale bar = 50 µm. Please click here to view a larger version of this figure.
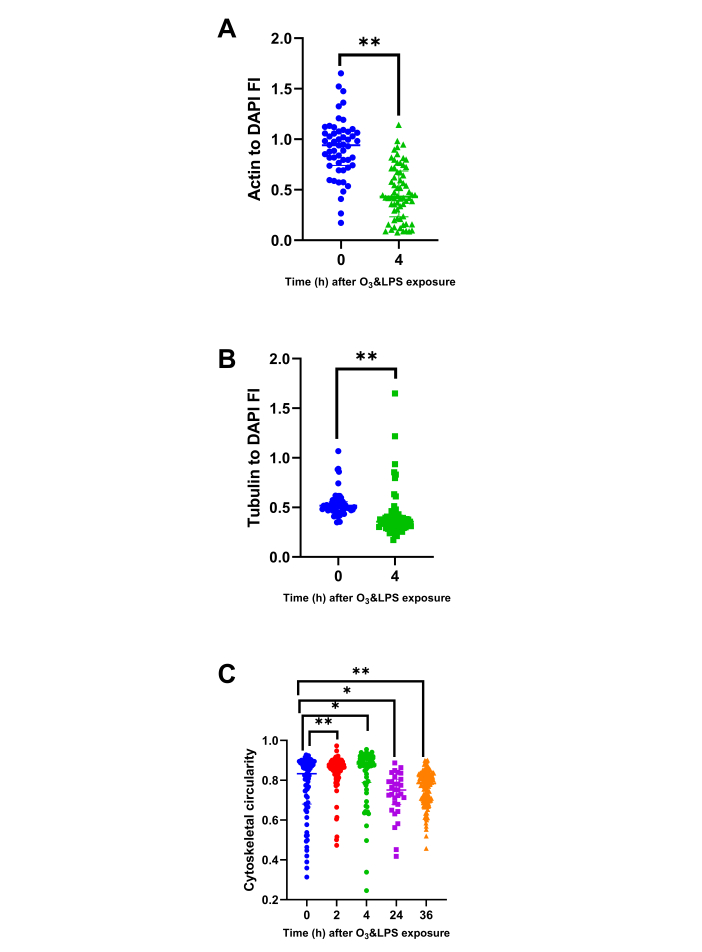
Figure 6: Bronchoalveolar lavage (BAL) cytoskeletal protein and circularity analysis: BAL cytospins stained for actin and tubulin were analyzed for DAPI normalized parameters i.e., A) Actin to DAPI fluorescent intensity (FI) ratio at 0 and 4 h, B) Tubulin to DAPI fluorescent intensity (FI) ratio at 0 and 4 h and C) BAL cell cellularity at 0 (n=75 cells), 2 (n=105 cells), 4 (n=66 cells), 24 (n=31 cells), 36 (n=154 cells) h. As a few pilot experiments were also performed immediately after O3 and LPS exposures i.e., at 2 h time-point, we also included some very early BAL cellularity analysis from the 2 h time-point to highlight the immediate effects of O3. For the graphs A-C, 0 h is represented in blue, 2 h in red, 4 h in green, 24 h in purple and 36 h in orange data points. * represents p<0.05, ** represents p<0.01. Please click here to view a larger version of this figure.

Figure 7: Bronchoalveolar lavage (BAL) intracellular and plasma membrane status: BAL cells were stained for vital stains, calcein (in green) and ethidium homodimers/EthHD (in red) as shown in representative upper image panels at A) 0, B) 24 and C) 36 h after O3 and LPS exposures. Scale bar = 200 µm. BAL cytospins were immunostained for DAPI (in blue), ATPα (in green) and Ly6G (in red). Representative images are shown in the lower image panels at A) 0 and B) 24 h after O3 and LPS exposures. Scale bar = 50 µm. Please click here to view a larger version of this figure.
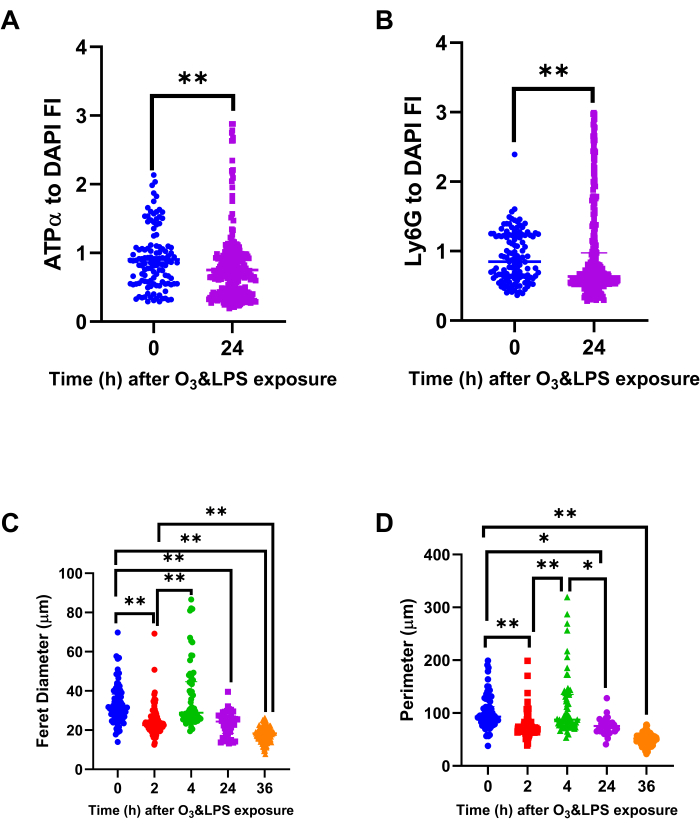
Figure 8: Bronchoalveolar lavage (BAL) mitochondrial protein ATPα, lysosomal protein Ly6G and cell size analysis: Immunostained BAL cells were normalized for DAPI to compute A) ATPα to DAPI fluorescent intensity (FI) ratio and B) Ly6G to DAPI FI ratio, at 0 and 24 h after O3 and LPS exposures. Immunostained BAL cells were also analyzed for their A) longest i.e., ferret diameter and B) perimeter, at 0, 2, 4, 24 and 36 h after O3 and LPS exposures. For the graphs A-D, 0 h (n=119 cells) is represented in blue, 2 h (n=105 cells) in red, 4 h (n=66 cells) in green, 24 h (n=309 cells) in purple and 36 h (n=154 cells) in orange data points. * represents p<0.05, ** represents p<0.01. Please click here to view a larger version of this figure.
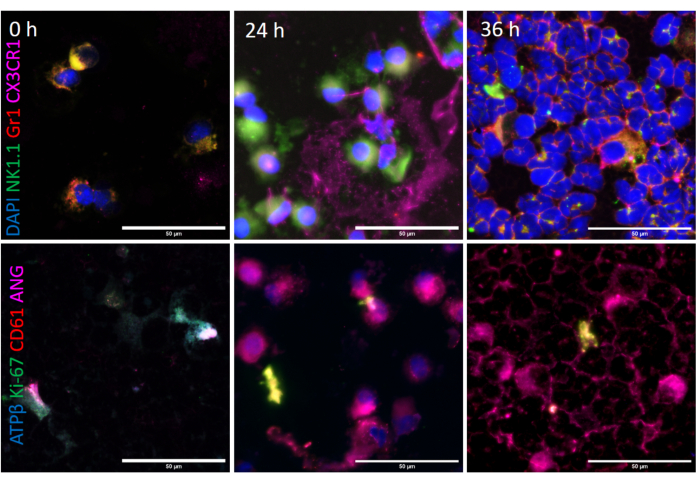
Figure 9: Bronchoalveolar lavage (BAL) intracellular phenotyping: BAL cells were immunostained for DAPI (in blue), NK1.1 (in green), Gr1 (in red) and CX3CR1 (in magenta) as shown in representative upper image panels at A) 0, B) 24 and C) 36 h after O3 and LPS exposures. Scale bar = 50 µm. BAL cytospins were immunostained for ATPβ (in blue), Ki-67 (in green), CD61 (in red) and angiostatin (in magenta). Representative images are shown in the lower image panels at A) 0, B) 24 and C) 36 h after O3 and LPS exposures. Scale bar = 50 µm. Please click here to view a larger version of this figure.
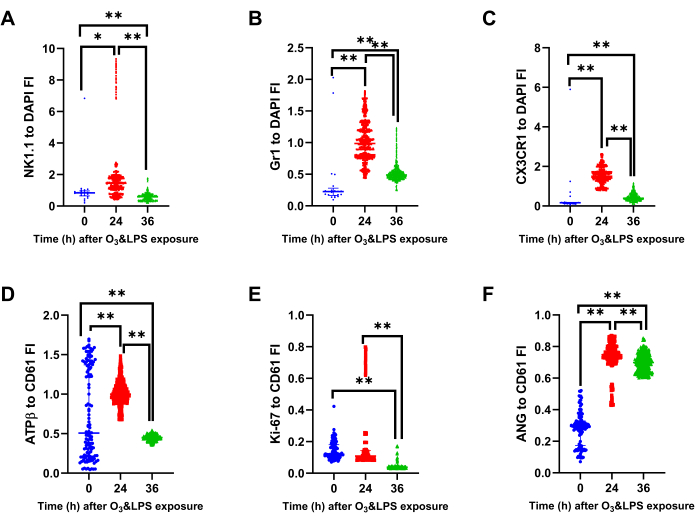
Figure 10: Bronchoalveolar lavage (BAL) mitochondrial protein ATPβ, lysosomal protein Gr1, intracellular CX3CR1 and angiostatin analysis: Immunostained BAL cells were normalized for DAPI to compute A) NK1.1 to DAPI fluorescent intensity (FI) ratio, B) Gr1 to DAPI FI ratio and C) CX3CR1 to DAPI FI ratio, at 0, 24 and 36 h after O3 and LPS exposures. Immunostained BAL cells were normalized for CD61 to compute A) ATPβ to DAPI fluorescent intensity (FI) ratio B) Ki-67 to DAPI FI ratio and B) Angiostatin (ANG) to DAPI FI ratio, at 0, 24 and 36 h after O3 and LPS exposures. For the graphs A-F, 0 h (n=21 cells) is represented in blue, 24 h (n=796 cells) in red and 36 h (n=2692 cells) in green data points. * represents p<0.05, ** represents p<0.01. Please click here to view a larger version of this figure.
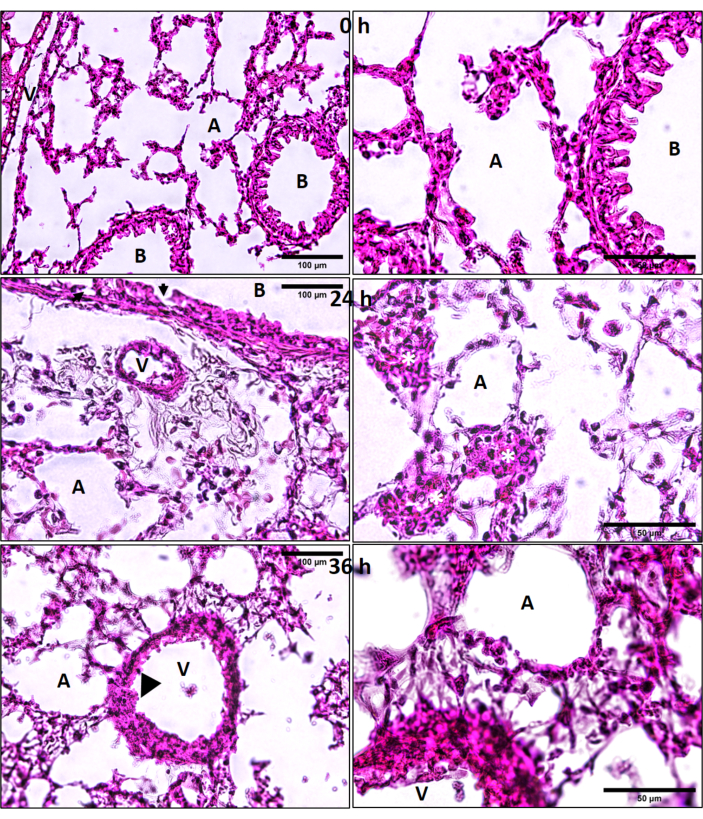
Figure 11: Lung hematoxylin and eosin (H&E) histology. Ozone (O3) and LPS induced lung cryosection H&E histology at 0, 24 and 36 h after combined O3 and LPS exposures. Regions of alveolar epithelial damage are marked by solid black arrows and endothelial damage by black arrow heads. Yellow asterisks (*) represent patches of leukocytes in the alveolar septal regions. A = alveolar space, B = bronchus, V = vasculature, scale bar = 100 µm for left hand image panels and 50 µm for image panels on the right side. Please click here to view a larger version of this figure.
| S.No. | Primary Antibody | Invitrogen Secondary Antibody |
| 1 | Mouse anti-NK1.1 IgG2a kappa (clone PK136), Invitrogen Catalog No. 16-5941-82 | Alexa 488 conjugated goat anti-mouse IgG (H+L), Catalog No. A11002 |
| 2 | Rat anti-Ly6G/Ly6C (Gr1) IgG2b kappa (clone RB6-8C5), Invitrogen Catalog No. 53-5931-82 | Alexa 568 conjugated goat anti-rat IgG (H+L), Catalog No. A11077 |
| 3 | Rabbit anti-CX3CR1 IgG (RRID 467880), Invitrogen Catalog No. 14-6093-81 | Alexa 633 conjugated goat anti-rabbit IgG (H+L), Catalog No. A21070 |
| 4 | Mouse anti-ATP5A1 IgG2b (clone 7H10BD4F9), Invitrogen Catalog No. 459240 | Alexa 488 conjugated goat anti-mouse IgG (H+L), Catalog No. A11002 |
| 5 | Rat anti-Ly6G IgG2a kappa (clone 1A8), Invitrogen Catalog No. 16-9668-82 | Alexa 568 conjugated goat anti-rat IgG (H+L), Catalog No. A11077 |
| 6 | Mouse anti-ATP5β IgG2b (clone 3D5AB1), Thermofisher Catalog No. A-21351 | Alexa 350 conjugated goat anti-mouse IgG (H+L), Catalog No. A11045 |
| 7 | Rat anti-Ki-67 (clone SolA15) IgG2a kappa, Invitrogen Catalog No. 14-5698-82 | Alexa 568 conjugated goat anti-rat IgG (H+L), Catalog No. A11077 |
| 8 | Armenian hamster anti-CD61 (clone 2C9.G2) IgG1 kappa, BD Catalog No. 553343 | Alexa 568 conjugated goat anti-hamster IgG (H+L), Catalog No. A21112 |
| 9 | Rabbit anti-angiostatin (mouse aa 98-116) IgG, Abcam Catalog No. ab2904 | Alexa 633 conjugated goat anti-rabbit IgG (H+L), Catalog No. A21070 |
Table 1: Primary and secondary antibody combinations used to stain cytospins prepared from the samples.
| Read-out | BAL | LVP | PB | SBM | FBM |
| TLC | * | * | + at 24 h; – at 36, 72 h | + at 72 h | |
| Neutrophils | + at 24, 36, 72 h | + at 24 h | * | + at 4, 24, 36, 72 h | + at 4, 24, 36, 72 h |
| Protein | + at 36 h | * | n.d. | n.d. | n.d. |
| Chemokine profile | – For eotaxin-2, IL-2 at 4, 24, 36, 72 h | + for Eotaxin-1/2, IL-2, TNFα, IFNγ, CX3CL1, CCL27, IL-16, CXCL5, CXCL10, MIP3β at 4 h; + for SDF1α at 36 h | n.d. | n.d. | n.d. |
| Cell size | + at 4 h; – at 24, 36 h | n.d. | n.d. | n.d. | n.d. |
| ATPα | – at 24 h | n.d. | n.d. | n.d. | n.d. |
| NK1.1/Ki-67 | + at 24 h | n.d. | n.d. | n.d. | n.d. |
| ATPβ/Gr1/CX3CR1/ANG | + at 24, 36 h | n.d. | n.d. | n.d. | n.d. |
Table 2: A comprehensive summary of the main findings of the study in different compartments. * denotes no change, + denotes an increase and – denotes a decrease in the measured parameters. BAL denotes broncho-alveolar lavage fluid, LVP denotes lung vascular perfusate, PB denotes peripheral blood, SBM denotes sternum bone marrow and FBM denotes femur bone marrow compartment.
Supplementary Figure 1: Gr1 and CD11b immunostaining: Representative images from DAPI (in blue), Gr1 (in green) and CD11b (in red) fluorescent immunostained cytospin slides of lung vascular perfusate (LVP), sternal bone marrow (BM) and femur bone marrow (BM) compartments at 0, 4 and 24 h after combined O3 and LPS exposures. Scale bar = 50 µm. Please click here to download this File.
Supplementary Movie 1 Three-dimensional rendered volume of BAL macrophages from 0 h time-point (i.e., baseline), showing nucleus (shown in blue) and immunostained for ATPα (shown in green) and Ly6G (shown in red). Please click here to download this Movie.
Supplementary Movie 2: Three-dimensional rendered volume of BAL macrophages from 24 h time-point after combined O3 and LPS exposures, showing nucleus (shown in blue) and immunostained for ATPα (shown in green) and Ly6G (shown in red). Note the presence of anuclear ATPα positive bodies as well as a fragmented cell. Please click here to download this Movie.
Discussion
The methods presented in the current study highlight the usefulness of multiple compartment analysis to study multiple cellular events during lung inflammation. We have summarized the findings in Table 2. We and many labs have extensively studied the murine response to intranasal LPS instillation, which is marked by rapid recruitment of lung neutrophils, which peaks between 6-24 h following which, resolution kicks in. And recently, we have shown that sub-clinical O3 (at 0.05 ppm for 2 h) alone can induce significant lung damage in the C57BL/6NJ sub-strain15, which is marked by partitioning of neutrophils in the lung vascular compartment, while damaged macrophages and debris were observed in the BAL, thus indicating the inflammatory potential of of O3. In that model we observed an absence of neutrophils in the alveolar compartment as a result of O3 induced alveolar epithelial damage and resulting phagocytosis by the alveolar macrophages. Not only did we observe extracellular DNA and debris in the O3 model, the BAL macrophages were dysmorphic which correlate with high IL-16 levels, an alarmin usually released by dying neutrophils. Therefore, it is important to understand whether these sub-clinical O3 levels can affect the immune response in otherwise robust C57BL/6J sub-strain, as noted with supra-physiological O3 levels21. We observed that combined O3 and intranasal bacterial endotoxin induced neutrophil recruitment in the pulmonary as well as systemic compartments, marked by presence of damaged neutrophils in BAL, lung vascular perfusate, sternal and femur bone marrow compartments starting at 4 h which remained high at 24, 36 and 72 h. The cellular damage was represented as loss of cortical actin and lamellipodia and increase in cell size in BAL leukocytes; neutrophils, analyzed in the BAL compartment, displayed significantly reduced positive reactivity to microtubule, ATP synthase complex V subunit α (ATPα) and active mitochondrial stain. Lungs adapt to this combined exposure by upregulating the expression of cellular ATP synthase complex V subunit β (ATPβ) as well as that of the ATPβ ligand, angiostatin (Table 2).
An important aspect of studying inflammation is to understand cellular necrosis along with chemokine gradients. It was interesting to note that the total leukocyte counts were not different except in peripheral blood and the femur bone marrow compartment. These observations led us to investigate the cellular cytoskeletal patterns, size and immunophenotyping. We observed a loss of lamellipodia and active mitochondria in BAL cells indicating cellular damage. Cortical actin organization is an essential feature of inter-cellular signaling and the resulting barrier properties. Cytoskeletal disorganization is thus a good marker of cell damage when necrosis is not as obvious to assess. Even at 36 h, the BAL cells had compromised plasma membrane as indicated by ethidium homodimer staining. Immediately after the combined exposures, neutrophils in BAL showed Gr1 positivity but an essentially negative staining for CD11b, which localizes towards the leading edge and aids in alveolar septal crawling22. Thus, the combined exposures lead to accumulation of atypical neutrophils devoid of the CD11b protein. LPS does not induce downregulation of CD11b. Thus, this feature is likely a result of O3 induced damage to the neutrophils.
The BAL cells had a unique protein signature with higher NK1.1, Ki-67 and ATPβ staining at 24 h compared to 0 h (Table 2). The BAL cells had higher expression of Gr1, CX3CR1 as well as angiostatin, at 24 as well as 36 h when compared to 0 h (Table 2). These findings were corroborated by the presence of progressively more Gr1 positive neutrophils in BAL, at 24 and 36 h, after exposure (Table 2). The presence of NK1.1, CX3CR1 and Gr1 positive cells, at 24 h, indicates the recruitment of both mononuclear and polymorphonuclear cells in the BAL. Amongst the mononuclear cells, the CX3CR1 positive cells dominated at 36 h indicating presence of either interstitial macrophages, platelets or monocyte derived macrophages. The inclusion of both Ki-67 and angiostatin as markers informed us about the proliferative vs anti-angiogenic milieu, respectively in the BAL. Thus, the combined O3 and LPS exposures lead to proliferation of probably macrophages followed by an anti-angiogenic milieu due to neutrophilic infiltration. The transient increase in ATPβ staining may indicate an important adaptation towards an increased survival of the BAL leukocytes at 24 h. It must be noted that ATPβ can bind to angiostatin and inhibit prolonged survival6,23, which is in fact reflected in the lower Ki-67 index at 36 h post exposure.
Although BAL protein was highest at 36 h, and not other time-points, the lung vascular perfusate did not show any changes in the protein concentration before or after exposure. It is possible that the vascular compartment was not affected but this is most likely confounded due to oxidation of electron-rich amino-acids such as methionine, histidine and cysteine by O3 induced free radicals and resultant surfactant proteins (SP-A, SP-B) degradation as well as vascular leak24,25,26. Quantification of BAL IgM, BAL surfactant proteins, BAL albumin or dye extravasation (using Evans blue or fluorescein iso-thiocyanate (FITC) dextran) are alternative methods to assess epithelial and endothelial integrity.
Interestingly, lung vascular perfusate levels of eotaxin-2 and IL-2 were acutely raised at the 4 h time-point. BAL eotaxin-2 and IL-2 levels were significantly reduced after exposure, indicating either degradation in the alveolar space or entrapment in the lung vasculature. More chemokines analysed in lung vascular perfusate revealed higher levels of the eosinophil chemokine (eotaxin-1), TNFα, IFNγ, lymph-node derived mononuclear cell chemokines (CX3CL1, CXCL10, CCL27, MIP3β), epithelial derived neutrophil chemokine (CXCL5), and the alarmin (IL-16) released after secondary necrosis of neutrophils27, but only at 4 h after exposure. At 36 h, the bone marrow derived pan-leukocyte chemokine, SDF1α28, was higher in the lung vascular perfusate. The bone marrow derived SDF1α concentrations correlate with cytology findings whereby CD11b and Gr1 positive cells are abundant at 24 and 36 h after exposure. Thus, the chemokine and cytological profiles reflect significant leukocyte mobilization from the bone marrow compartments (Table 2).
Our animal model and research findings are vital keeping in mind the real world situations whereby living beings in urban settings are likely exposed to such sub-clinical O3 and infectious agents8. Our murine model serves as a readily accessible prototype that can be reproduced in level-2 containment labs for studying pulmonary and extra-pulmonary mechanisms of invasive cell death and infections, as is case with COVID-19 infections29. Future studies should be directed at visualizing the repair or late phase follow-up as well as chronic exposures to investigate the long-term cellular adaptations. Thus, for comprehensive lung inflammation studies involving live organ15 and animal16,30,31 imaging techniques, the current end-point study design can provide vital insights into the host response to combined low-dose O3 (cell death) and LPS (immune stimulation), and thus deciphering the mechanisms of acute lung injury.
Divulgations
The authors have nothing to disclose.
Acknowledgements
The research conducted is funded by President's NSERC grant as well as start-up funds from the Sylvia Fedoruk Canadian Center for Nuclear Innovation. The Sylvia Fedoruk Canadian Center for Nuclear Innovation is funded by Innovation Saskatchewan. Fluorescence imaging was performed at the WCVM Imaging Centre, which is funded by NSERC. Jessica Brocos (MSc Student) and Manpreet Kaur (MSc Student) were funded by the start-up funds from the Sylvia Fedoruk Canadian Center for Nuclear Innovation.
Materials
| 33-plex Bioplex chemokine panel | Biorad | 12002231 | |
| 63X oil (NA 1.4-0.6) Microscope objectives | Leica | HCX PL APO CS (11506188) | |
| Alexa 350 conjugated goat anti-mouse IgG (H+L) | Invitrogen | A11045 | |
| Alexa 488 conjugated goat anti-mouse IgG (H+L) | Invitrogen | A11002 | |
| Alexa 488 conjugated phalloidin | Invitrogen | A12370 | |
| Alexa 555 conjugated mouse anti-α tubulin clone DM1A | Millipore | 05-829X-555 | |
| Alexa 568 conjugated goat anti-hamster IgG (H+L) | Invitrogen | A21112 | |
| Alexa 568 conjugated goat anti-rat IgG (H+L) | Invitrogen | A11077 | |
| Alexa 633 conjugated goat anti-rabbit IgG (H+L) | Invitrogen | A21070 | |
| Armenian hamster anti-CD61 (clone 2C9.G2) IgG1 kappa | BD Pharmingen | 553343 | |
| C57BL/6 J Mice | Jackson Laboratories | 64 | |
| Confocal laser scanning microscope | Leica | Leica TCS SP5 | |
| DAPI (4′,6-diamidino-2-phenylindole) | Invitrogen | D1306 | aliquot in 2 µl stocks and store at -20°C |
| Inverted fluorescent wide field microscope | Olympus | Olympus IX83 | |
| Ketamine (Narketan) | Vetoquinol | 100 mg/ml | Dilute 10 times to make a 10 mg/ml stock |
| Live (calcein)/Dead (Ethidium homodimer-1) cytotoxicity kit | Invitrogen | L3224 | |
| Mouse anti-ATP5A1 IgG2b (clone 7H10BD4F9) | Invitrogen | 459240 | |
| Mouse anti-ATP5β IgG2b (clone 3D5AB1) | Invitrogen | A-21351 | |
| Mouse anti-NK1.1 IgG2a kappa (clone PK136) | Invitrogen | 16-5941-82 | |
| Pierce 660 nm protein assay | Thermoscientific | 22660 | |
| Rabbit anti-angiostatin (mouse aa 98-116) IgG | Abcam | ab2904 | |
| Rabbit anti-CX3CR1 IgG (RRID 467880) | Invitrogen | 14-6093-81 | |
| Rat anti-Ki-67 (clone SolA15) IgG2a kappa | Invitrogen | 14-5698-82 | |
| Rat anti-Ly6G IgG2a kappa (clone 1A8) | Invitrogen | 16-9668-82 | |
| Rat anti-Ly6G/Ly6C (Gr1) IgG2b kappa (clone RB6-8C5) | Invitrogen | 53-5931-82 | |
| Rat anti-mouse CD16/CD32 Fc block (clone 2.4G2) | BD Pharmingen | 553142 | |
| Reduced mitotracker orange | Invitrogen | M7511 | |
| Xylazine (Rompun) | Bayer | 20 mg/ml | Dilute 2 times to make a 10 mg/ml stock |
References
- Bhattacharya, J., Matthay, M. A. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annual Review of Physiology. 75, 593-615 (2013).
- Aulakh, G. K. Neutrophils in the lung: “the first responders”. Cell Tissue Research. , (2017).
- Aulakh, G. K., Suri, S. S., Singh, B. Angiostatin inhibits acute lung injury in a mouse model. American Journal of Physiology – Lung Cellular and Molecular Physiology. 306 (1), 58-68 (2014).
- Schneberger, D., Aulakh, G., Channabasappa, S., Singh, B. Toll-like receptor 9 partially regulates lung inflammation induced following exposure to chicken barn air. Journal of Occupational Medicine and Toxicology. 11 (1), 1-10 (2016).
- Shah, D., Romero, F., Stafstrom, W., Duong, M., Summer, R. Extracellular ATP mediates the late phase of neutrophil recruitment to the lung in murine models of acute lung injury. American Journal of Physiology – Lung Cellular and Molecular Physiology. 306 (2), 152-161 (2014).
- Aulakh, G. K., Balachandran, Y., Liu, L., Singh, B. Angiostatin inhibits activation and migration of neutrophils. Cell Tissue Research. , (2013).
- Cakmak, S., et al. Associations between long-term PM2.5 and ozone exposure and mortality in the Canadian Census Health and Environment Cohort (CANCHEC), by spatial synoptic classification zone. Environment International. 111, 200-211 (2018).
- Dauchet, L., et al. Short-term exposure to air pollution: Associations with lung function and inflammatory markers in non-smoking, healthy adults. Environment International. 121, 610-619 (2018).
- Delfino, R. J., Murphy-Moulton, A. M., Burnett, R. T., Brook, J. R., Becklake, M. R. Effects of air pollution on emergency room visits for respiratory illnesses in Montreal, Quebec. American Journal of Respiratory and Critical Care Medicine. 155 (2), 568-576 (1997).
- Peterson, M. L., Harder, S., Rummo, N., House, D. Effect of ozone on leukocyte function in exposed human subjects. Environmental Research. 15 (3), 485-493 (1978).
- Rush, B., et al. Association between chronic exposure to air pollution and mortality in the acute respiratory distress syndrome. Environmental Pollution. 224, 352-356 (2017).
- Rush, B., Wiskar, K., Fruhstorfer, C., Celi, L. A., Walley, K. R. The Impact of Chronic Ozone and Particulate Air Pollution on Mortality in Patients With Sepsis Across the United States. Journal of Intensive Care Medicine. , (2018).
- Stieb, D. M., Burnett, R. T., Beveridge, R. C., Brook, J. R. Association between ozone and asthma emergency department visits in Saint John, New Brunswick, Canada. Environmental Health Perspectives. 104 (12), 1354-1360 (1996).
- Thomson, E. M., Pilon, S., Guenette, J., Williams, A., Holloway, A. C. Ozone modifies the metabolic and endocrine response to glucose: Reproduction of effects with the stress hormone corticosterone. Toxicology and Applied Pharmacology. 342, 31-38 (2018).
- Aulakh, G. K., Brocos Duda, J. A., Guerrero Soler, C. M., Snead, E., Singh, J. Characterization of low-dose ozone-induced murine acute lung injury. Physiological Reports. 8 (11), 14463 (2020).
- Aulakh, G. K., et al. Quantification of regional murine ozone-induced lung inflammation using [18F]F-FDG microPET/CT imaging. Scientific Reports. 10 (1), 15699 (2020).
- Charavaryamath, C., Keet, T., Aulakh, G. K., Townsend, H. G., Singh, B. Lung responses to secondary endotoxin challenge in rats exposed to pig barn air. Journal of Occupational Medicine and Toxicology. 3, 24 (2008).
- Szarka, R. J., Wang, N., Gordon, L., Nation, P. N., Smith, R. H. A murine model of pulmonary damage induced by lipopolysaccharide via intranasal instillation. Journal of Immunological Methods. 202 (1), 49-57 (1997).
- Southam, D. S., Dolovich, M., O’Byrne, P. M., Inman, M. D. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. American Journal of Physiology – Lung Cellular and Molecular Physiology. 282 (4), 833-839 (2002).
- Aulakh, G. K. Lack of CD34 produces defects in platelets, microparticles, and lung inflammation. Cell Tissue Research. , (2020).
- Gilmour, M. I., Hmieleski, R. R., Stafford, E. A., Jakab, G. J. Suppression and recovery of the alveolar macrophage phagocytic system during continuous exposure to 0.5 ppm ozone. Experimental Lung Research. 17 (3), 547-558 (1991).
- Yipp, B. G., et al. The Lung is a Host Defense Niche for Immediate Neutrophil-Mediated Vascular Protection. Science Immunology. 2 (10), (2017).
- Lee, T. Y., et al. Angiostatin regulates the expression of antiangiogenic and proapoptotic pathways via targeted inhibition of mitochondrial proteins. Blood. 114 (9), 1987-1998 (2009).
- Hawkins, C. L., Davies, M. J. Detection, identification, and quantification of oxidative protein modifications. Journal of Biological Chemistry. 294 (51), 19683-19708 (2019).
- Hemming, J. M., et al. Environmental Pollutant Ozone Causes Damage to Lung Surfactant Protein B (SP-B). Biochimie. 54 (33), 5185-5197 (2015).
- Oosting, R. S., et al. Exposure of surfactant protein A to ozone in vitro and in vivo impairs its interactions with alveolar cells. American Journal of Physiology. 262 (1), 63-68 (1992).
- Roth, S., et al. Secondary necrotic neutrophils release interleukin-16C and macrophage migration inhibitory factor from stores in the cytosol. Cell Death & Discovery. 1, 15056 (2015).
- Kawaguchi, N., Zhang, T. T., Nakanishi, T. Involvement of CXCR4 in Normal and Abnormal Development. Cells. 8 (2), (2019).
- Gupta, A., et al. Extrapulmonary manifestations of COVID-19. Nature Medicine. 26 (7), 1017-1032 (2020).
- Aulakh, G. K., Kuebler, W. M., Singh, B., Chapman, D. . 2017 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC). , 1-2 (2017).
- Aulakh, G. K., et al. Multiple image x-radiography for functional lung imaging. Physics in Medicine & Biology. 63 (1), 015009 (2018).

