Generation of Human Neurons and Oligodendrocytes from Pluripotent Stem Cells for Modeling Neuron-Oligodendrocyte Interactions
Summary
The neuron-glial interactions in neurodegeneration are not well understood due to inadequate tools and methods. Here, we describe optimized protocols to obtain induced neurons, oligodendrocyte precursor cells, and oligodendrocytes from human pluripotent stem cells and provide examples of the values of these methods in understanding cell-type-specific contributions in Alzheimer’s disease.
Abstract
In Alzheimer’s disease (AD) and other neurodegenerative disorders, oligodendroglial failure is a common early pathological feature, but how it contributes to disease development and progression, particularly in the gray matter of the brain, remains largely unknown. The dysfunction of oligodendrocyte lineage cells is hallmarked by deficiencies in myelination and impaired self-renewal of oligodendrocyte precursor cells (OPCs). These two defects are caused at least in part by the disruption of interactions between neuron and oligodendrocytes along the buildup of pathology. OPCs give rise to myelinating oligodendrocytes during CNS development. In the mature brain cortex, OPCs are the major proliferative cells (comprising ~5% of total brain cells) and control new myelin formation in a neural activity-dependent manner. Such neuron-to-oligodendrocyte communications are significantly understudied, especially in the context of neurodegenerative conditions such as AD, due to the lack of appropriate tools. In recent years, our group and others have made significant progress to improve currently available protocols to generate functional neurons and oligodendrocytes individually from human pluripotent stem cells. In this manuscript, we describe our optimized procedures, including the establishment of a co-culture system to model the neuron-oligodendrocyte connections. Our illustrative results suggest an unexpected contribution from OPCs/oligodendrocytes to the brain amyloidosis and synapse integrity and highlight the utility of this methodology for AD research. This reductionist approach is a powerful tool to dissect the specific hetero-cellular interactions out of the inherent complexity inside the brain. The protocols we describe here are expected to facilitate future studies on oligodendroglial defects in the pathogenesis of neurodegeneration.
Introduction
Oligodendrocyte lineage cells—including oligodendrocyte precursor cells (OPCs), myelinating oligodendrocytes, and transitional types in between—constitute a major group of human brain cells1 that actively participate in many critical functions for the proper operation and maintenance of our central nervous system throughout neural development and aging2,3,4. While oligodendrocytes are well known for producing myelin to facilitate neuronal activity transmission and support axonal health in white matter, OPCs are abundant (~5%) in gray matter where myelination is scarce and perform activity-dependent signaling functions to govern learning behavior and memory formation5,6,7,8. How oligodendroglial cells function and dysfunction in the pathogenesis of Alzheimer’s disease (AD) and other age-associated neurodegenerative conditions has been understudied9. The inadequacies of an appropriate model system and deficiencies in general knowledge to guide an experimental path forward are the major reasons for this gap.
In light of the latest breakthroughs in deriving human brain cells from pluripotent stem cells including embryonic stem (ES) and induced pluripotent stem (iPS) cells, such cellular models in conjunction with modern gene editing tools have emerged as robust tools to handle the intricate nexus of cellular interactions in the brain, and are capable of demonstrating human-specific disease manifestations10,11. Considering that individual brain cell types can exhibit distinct and even conflicting effects in the face of the same AD-promoting conditions12,13, this stem cell methodology uniquely offers cell type-specific information that has previously been missed using established in vivo or in vitro models that only provide aggregate readouts from collections of brain cell types. In the last decade, a good number of reliable protocols have been developed to generate human neurons from trans-differentiation of ES/iPS cells or direct conversion from other terminally differentiated cell types (e.g., fibroblasts)14,15. In particular, the application of key neurogenic transcription factors (e.g., neurogenin 2, Ngn2)16 to human pluripotent stem cells can generate a homogeneous population of well-characterized neuronal cell types for pure cultures without a need for coculturing with glial cells12,17,18. For induced human oligodendrocytes, there are a few published protocols that can generate functional cells highly resembling their primary counterparts, with a wide range of efficiency and demand in time and resources19,20,21,22,23,24,25,26,27,28. To date, none of these protocols have been applied to investigate how oligodendroglial cells respond to and affect AD pathogenesis.
Here, we describe our improved protocols for single and mixed cultures of human induced neurons (iNs) and OPCs/oligodendrocytes (iOPCs/iOLs). The iN protocol described here is based on the widely used Ngn2 approach16, and has the additional feature of being glia-free. The resultant iNs are homogenous and highly resemble the cortical layer 2/3 excitatory neurons, with characteristic pyramidal morphology, gene expression pattern and electrophysiological features17,18 (Figure 1). To overcome some of the fundamental barriers in directed differentiation of pluripotent stem cells, we have developed a simple and effective method of low-dose dimethyl sulfoxide (DMSO) pre-treatment29,30, and reported an enhanced propensity of human ES/iPS cells to transdifferentiate into iOPCs and iOLs31, based on a widely-adapted protocol by Douvaras and Fossati32. We have further simplified the protocol and incorporated a robust differentiation-promoting compound, clemastine7,33,34, to accelerate the process of oligodendroglial maturation. As a result (Figure 2), the iOPCs can be generated in 2 weeks (~95% positive for the marker O4) and iOLs in four weeks (expressing mature markers MBP and PLP1). Interestingly, we found iOPCs alone secrete a remarkable amount of amyloid-β (Aβ), consistent with the independent transcriptomic data showing the abundant expression of the amyloid precursor protein (APP) and the processing protease β-secretase (BACE1) in oligodendrocyte lineage cells35,36. Moreover, our iN-iOPC co-culture system promotes the ensheathing of axons by MBP-positive iOL processes and provides significant support for synapse formation (Figure 3). Thus, the protocols we have detailed below have technical and biological advantages over previously catalogued neuron-oligodendroglia co-culturing methods, and hold a promise in better modeling the neurodegeneration in AD.
Protocol
1. Human neuron induction from human pluripotent stem cells
- Lentivirus preparation (~5 days, detailed protocol as described previously16)
- Plate ~1 million HEK293T cells each T75 flask, to have them ~40% confluent when performing transfection. Transfect them with plasmids expressing tetracycline-inducible Ngn2 and puromycin-resistant gene (PuroR; under the same TetO promoter control), rtTA and the three helper plasmids pRSV-REV, pMDLg/pRRE, and VSV-G (12 µg of lentiviral vector DNA and 6 µg of each of the helper plasmid DNA). Prepare at least three flasks per lentivirus preparation. Use PEI for transfection following the manufacturer’s instruction. Change the media after 16 h and discard.
- Harvest released viral particles by collecting culture media every day and replace with fresh media for 3 days. Pool the collected media containing viral particles for purification. Filter the virus through a 0.22 µm filter and centrifuge at 49,000 x g for 90 min. Resuspend the pellet in the appropriate volume of PBS-glucose (~150 µL).
- Neuron Induction (~5 days)
NOTE: This induction protocol (Figure 1A; flow diagram) is highly effective for both iPS and ES cells of validated pluripotency (which can be assayed by immunohistochemistry staining of well-characterized pluripotency markers; Figure 1B).- Use commercially available H1 human ES cells at the passage of 52 (see Table of Materials). Culture the cells on extracellular matrix solution coated 6-well plates (~0.5 mg of matrix solution per 6-well plate; see Table of Materials) using ES cell maintenance medium (see Table of Materials) and incubate the plates at 37 °C with 5% CO2.
- On Day -2, detach ES cells (80% confluent) with 1 mL of cell detachment solution (see Table of Materials) and incubate at room temperature for 10 min. Transfer the cells to a tube; wash the well with 2 mL of media and combine in the same tube. Centrifuge at 300 x g for 5 min, resuspend the pellet in media, and plate the cells onto matrix coated 6-well plates at the seeding density of 1 x 105 cells per well.
- On Day -1, add lentiviruses expressing Ngn2 plus PuroR and rtTA together with polybrene (8 µg/ml) to the ES cells in fresh ES cell maintenance medium (see Table of Materials). The exact amount of viruses should be determined by actual titers or the titration. We typically add 5 µL each virus per well in a 6-well plate.
- On Day 0, add Doxycycline (2 µg/mL, to activate Ngn2 expression) in DMEM-F12 medium with N2 supplement without morphogens.
- On Day 1, add Puromycin in fresh medium of DMEM-F12 plus N2 and doxycycline, to the final concentration of 1 µg/mL medium. Select the transduced cells in Puromycin for at least 24 h. Higher Puromycin concentration (up to 5 µg/mL) and longer selection period (up to 48 h) may be required to adequately remove the under-transduced cells if the virus titer is low.
- On Day 2, detach differentiating neurons with cell detachment solution (see Table of Materials), and re-plate them on 24-well plates (between 80,000–200,000 cells/well) coated with matrix solution (see Table of Materials), and maintain them in NBA/B27 medium without doxycycline. The seeding density is critical.
- At this stage, detached neurons can be frozen in specialized commercial freezing medium (see Table of Materials) and stored in liquid nitrogen for up to 3 months. Pure neurons can be plated accounting for the typical ~15%–20% cell death post-thaw, cultured alone or co-cultured with other brain cell types (see step 3.2.3. for co-culturing with OPCs).
- Culture pure iNs on the plates coated with extracellular matrix-based solutions as instructed by the manufacturer (see Table of Materials). The characteristic pyramidal morphology should be apparent by Day 4 (and Day 6; Figure 1C). The synapse formation can be detected as early as Day 14 to 16 and is prominent at Day 24 by immunohistochemical staining with standard pre- and post-synaptic markers. (Figure 1D; labeled with the pre-synaptic marker Synapsin 1 and the dendritic marker Map2).
2. Human oligodendrocyte precursor cell (OPCs) induction from pluripotent stem cells and oligodendrocyte maturation
- Neural Progenitor Cell (NPC) generation: monolayer protocol (~7 days). See Figure 2A for the flow diagram.
- Culture H1 human ES cells as described earlier (see step 1.2.1.) and trans-differentiate them into neural progenitor cells (NPCs) by an established approach called dual SMADi, with small molecule inhibitors for multiple signaling pathways. Here we use a widely accepted commercial kit and follow the monolayer protocol provided by the manufacturer (see Table of Materials).
- On Day -1, plate 0.5–1 x 106 cells per well in a 6-well plate coated by a growth factor reduced matrix solution (see Table of Materials; ~0.5 mg of matrix solution per 6-well plate) with ES cell maintenance medium (see Table of Materials). This growth factor reduced matrix solution is used to coat all the plates that will be used in the following steps.
- On Day 0, treat cells for 24 h with ES cell maintenance medium (see Table of Materials) supplemented by 2% DMSO.
- On Day 1–6, change the full media with warm (37 °C) neural induction medium containing the SMAD inhibitors from the commercial kit (see Table of Materials). If cells divide and reach confluence before Day 7, passage them to the seeding density of 0.5–1 x 106, as described earlier in step 2.1.2.
- On Day 7, passage NPCs using cell detachment solution (see Table of Materials) and plate at a seeding density of 1–2 x 105 cells/well of a 24-well plate.
- Assay the differentiation efficiency by immunohistochemical (IHC) staining for absence of pluripotency marker, OCT4 for example, and presence of NPC markers such as PAX6, Nestin, and Sox1.
- At this stage, detached NPCs can be frozen in the specialized commercial NPC freezing media (see Table of Materials) and stored in liquid nitrogen for up to 3 months. After freeze-and-thaw for once, NPCs still retain the multipotency to give rise to neurons, astrocytes, and OPCs with reliable protocols.
- Oligodendrocyte precursor cell (OPC) generation (~7 days). Please see Figure 2A for the flow diagram.
- On Day 7, passage NPCs using cell detachment solution (see Table of Materials) and plate them at a seeding density of 1–2 x 105 cells per well in a 24-well plate in warm (37 °C) neural induction medium plus SMAD inhibitors from the commercial kit (see Table of Materials).
- On Day 8, prepare a solution of 1% DMSO in the OPC differentiation medium and treat the plated NPCs for 24 h. The OPC differentiation medium is composed of: DMEM/F12 medium, 1% N2 supplement, 1% B27 supplement, bFGF at 20 ng/mL, SAG at 1 µM, PDGF-AA at 10 ng/mL (see Table of Materials).
- On Day 9, replace media with fresh OPC differentiation medium without DMSO. Feed the cells every other day until Day 15. If the cells reach confluence before Day 15, passage them to the seeding density of 1–2 x 105 cells per well as described in step 2.2.1.
- On Day 14, plate OPCs in OPC differentiation medium at a density of 1–2 x 105 cells/well in a 24-well plate.
- At this stage (Day 15), test cells for the presence of OPC-specific markers by IHC staining or qPCR (e.g., O4, Olig1/2, CSPG4/Ng2, NKX2.2, PDGFRa; Figure 2B) and for the absence of NPC markers (Pax6 or Nestin; Figure 2D). We typically detect the O4 immunoreactivity in more than 95% of the cells at Day 15. Of particular relevance to Alzheimer’s disease, the expression of APP (amyloid precursor protein), BACE1 (the processing protease β-secreatase 1), and peptide amyloid-β (Aβ) is abundant in OPCs (Figure 2F).
- Oligodendrocyte (OL) maturation (~7–20 days)
- On Day 15, replace media with OL maturation medium: Neurobasal-A medium, 2% B27 supplement, 1 µM cAMP, 200 ng/mL T3 triiodothyronine, and Clemastine of 1 µM (see Table of Materials). Change the medium every other day or every day, if necessary.
- When cells reach 90% confluence, split at a 1:3 ratio up to 2 passages or until cell division slows down substantially. If OPCs divide too fast and reach confluency in less than 3 days, add Ara-C (see Table of Materials) at a concentration of 2–5 µM for 1–3 days. Active proliferation indicates lowered maturation efficiency.
- Examine the efficiency of oligodendroglial maturation by assessing the expression of OL markers, e.g., CLDN11, PLP1, MBP by qPCR, IHC staining or immunoblotting. The characteristic morphology of highly complex structures (Figure 2C) and the expression of OL markers (Figure 2E) should be readily detected by Day 28.
3. Co-culturing of human induced neurons (iNs) and oligodendrocyte precursor cells (iOPCs)
- iOPC plating (~3 days)
- Plate iOPCs at Day 14 at a density of 1 x 105 cells per well in a 24-well plate (as described above in step 2.2.4.) in OPC differentiation medium (as described in step 2.2.2.).
- iN-iOPC co-culture set up
- On Day 15, detach the induced human neurons at the step of Day 2 after the Puromycin selection (as described in step 1.2.6.) with cell detachment solution (see Table of Materials).
- Add neurons onto the cultured OPCs, plating at the seeding density of 2 x 105 cells per well in the 24-well plate with growing OPCs (from step 3.1.1). Use the co-culture medium containing Neurobasal-A medium, 2% B27 supplement, and 100 ng/mL T3 triiodothyronine. Change the medium on the next day and then every other day afterwards. If OPCs proliferate too fast and reach confluency in less than 3 days, add Ara-C at a concentration of 2–5 µM. A representative image of the iNs and iOPCs grown in co-culture after 7 days neurons is shown in Figure 3A.
- Use frozen neurons prepared as described above in step 1.2.7 for co-culturing with OPCs. Plate freeze-and-thaw neurons at a higher density of 3 x 105 cells per well.
- After Day 14–16 in co-cultures, the synapse formation in iNs can be observed by IHC staining of pre- and post-synaptic markers, and by Day 21 the synaptic puncta should be abundant (Figure 3C) and neuronal activities can be reliably recorded.
- Starting at Day 21, test cells for OL specific markers (for example, MBP and PLP1). By Day 28, we normally observe the phenomenon ensheathing of iN axons by iOL processes, labeled by IHC staining for specific markers (Figure 3B; neurofilament NF for iN axons and MBP for iOPC processes).
Representative Results
Direct generation of human induced neurons from human pluripotent stem cells
It is very important that the starting human pluripotent stem cells exhibit a high degree of pluripotency for successful generation of iNs or iOPCs/iOLs. Therefore, cells should be stained for specific markers, such as Oct4 and SOX2, before starting either of the induction protocols described in the present manuscript (Figure 1A). Human H1 cells were used to obtain induced excitatory forebrain neurons following the previously published protocol by Zhang et al. with some modifications (Figure 1C)12,16,17,18. Here, we present a protocol in which iNs at Day 2 are re-plated in pure culture on matrix solution (see Table of Materials), in the absence of any feeder layer: glia or fibroblasts. In addition to the previously published protocols, we observe that freezing iNs at Day 2 does not significantly affect cell viability (~15%–20% cell death after thawing). Pure neurons in culture will start expressing synapsin1 at Day 14–16 (Figure 1D). Establishing a pure neuronal culture is very important because certain factors, for example, the leading AD risk factor ApoE, can be expressed by cells in the feeder layer and this can significantly confound the results.
iOPC generation and iOL maturation is improved by DMSO treatment
Here, we present a fast and efficient protocol that enables the generation of iOPCs in 2 weeks and mature iOLs in 4–5 weeks (Figure 2A). We leveraged the method of transient DMSO treatment we previously developed to augment the differentiation efficiency for ES and iPS cells29,30,31. DMSO treatment enriches the number of cells in the early G1 phase for better signaling integration, favoring differentiation. We performed the first treatment before inducing human ES cells to generate NPCs, and the second treatment before differentiating NPCs into iOPCs. We can detect specific OPC markers (Olig2, CSPG4, NKX2.2, and PDGFRA) as early as 2 weeks after plating of ES H1 cells (Figure 2B,E). The iOPC population at this stage is fairly homogenous, with >95% of cells positive for O4 staining and a high level of immunoreactivity for other markers (Figure 2B). After the start of OL maturation at Day 15, we can typically detect specific OL markers (MBP, O1, CLDN11, and PLP1) starting at Day 28 (Figure 2C,E). The expression of these stage-specific markers correlates with the developmental course of oligodendroglial cells and suggests an accelerated pace, with the NPC markers progressively going down, OPC markers peaking around the second week, and OL markers elevating by the third week (Figure 2D,E)37. Please note that this maturation process diversifies the cell populations. The subpopulations in continuum, comprising multiple intermediate stages between OPCs and mature myelinating oligodendrocytes, can be present and account for varying percentage of total cells, with more mature cells dominating at a later time.
As a comparison, we purchased the highly referenced iOPCs, and matured them in iOL following the manufacturer protocol. We tested the expression of the markers mentioned above in both our iOPC and iOL preparations, and in the cells we purchased. We determined that the cells generated following our protocol had higher expression of all the genes tested (Figure 2E). Interestingly, when we tested the secreted levels of two major isoforms of amyloid-β (Aβ40 and Aβ42) in iNs versus iOPCs, we noticed that iOPCs secreted more of both fragments, but the ratio remained the same (Figure 2F).
Co-culturing of iNs and iOPCs
This protocol is optimized specifically for co-culturing iNs and iOPCs and allow our real-time monitoring of the inter-cellular communications between these two cell types along the course of neural development. The ideal plating densities for both cell types need to be decided with a series of cell number titration to achieve proper differentiation (Figure 3A). After 4 weeks in co-cultures, the iOPCs are expected to be adequately differentiated into OLs that are positive for specific markers such as MBP and extend processes to ensheath axons (Figure 3B). The co-culture system can robustly boost up the number of synapses, indicating that the iOPCs provide a neuronal support through physical contacts or release of trophic factors (Figure 3C). We can maintain the co-cultures in acceptable health condition for up to 6 weeks and observe that the synapse number and other neuronal attributes plateau around the fifth week. Of note, astrocytes and microglia are not present in our preparations and their absence can be documented by checking the expression of specific markers (Figure 3D). The iOPCs express a good number of well-characterized genes that can potentially respond to and mediate the activity-dependent signals from neighboring neurons, in a paracrine (e.g. neurotrophins and metabolites) and/or a synaptic manner (Figure 3E and 3F).
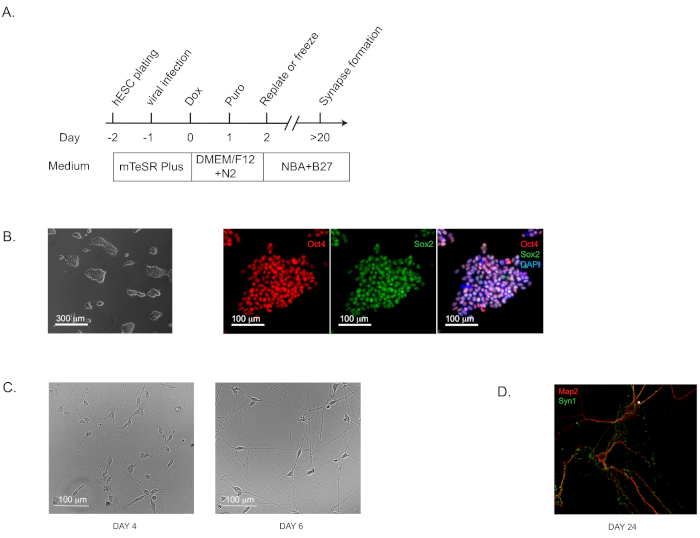
Figure 1: Direct Generation of human induced neurons (iNs) from hPSCs. (A) Flow diagram of iN generation. (B) Representative bright field and immunofluorescence images of the starting culture of human pluripotent stem cells (H1) to confirm the pluripotency. Oct4 is shown in red and Sox2 in green. (C) Representative bright field images of iNs at Day 4 and Day 6. (D) The characteristic morphology for dendritic arborization and synapse puncta in iNs grown in pure culture for 24 days and stained by immunofluorescence staining for dendritic marker Map2 and pre-synaptic marker Synapsin 1 (Syn1). Please click here to view a larger version of this figure.
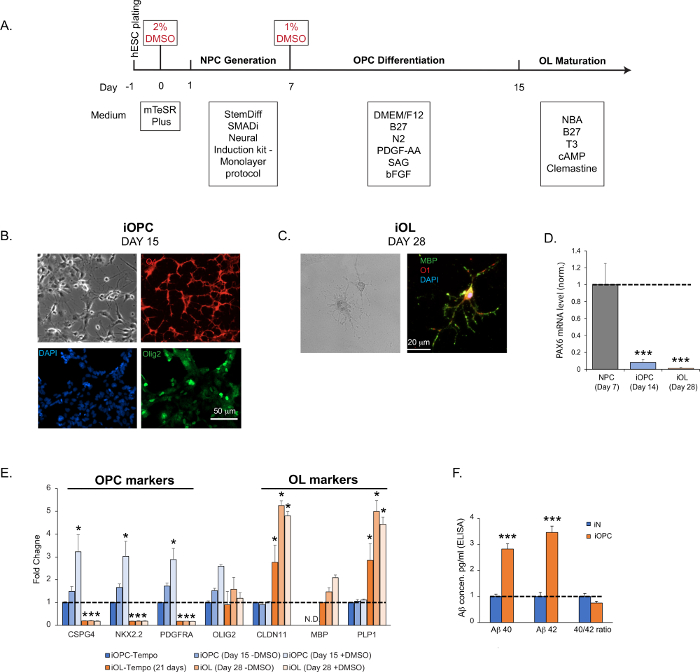
Figure 2: iOPC generation and iOL maturation. (A) Flow diagram of iOPC and iOL generation. (B) Representative bright field and immunofluorescence images of iOPCs at Day 15. Olig2 (pan-oligodendroglia marker) is shown in green, O4 (OPC marker) in red, and DAPI in blue. The imaging revealed that >95% of iOPCs are positive for O4 and 25% for Olig2. (C) Representative bright field and immunofluorescence images of iOLs at Day 28. MBP is shown in green, O1 in red, and DAPI in blue. (D) The expression of NPC marker PAX6 diminishes dramatically in iOPCs at Day 14 and further lowers to background in OLs at Day 28, indicating a robust NPC trans-differentiation and a high level of homogeneity in the iOPC population. (E) The time-course expression profile of common OPC and OL marker genes in cultures generated by the described protocol, without (-DMSO) or with (+DMSO) the step of DMSO incubation (steps 2.1.3 and 2.2.2), assayed at different time points. As a comparison, commercial iOPCs (see Table of Materials) were matured according to the manufacturer’s instructions, and both iOPCs (iOPC-Tempo) or iOLs (iOL-Tempo) were tested for the same markers. As expected, MBP (a mature oligodendrocyte marker) was not detected (N.D.) at the early stages of differentiation in all the iOPCs tested. The DMSO significantly enhanced the efficiency of OPC differentiation and OL maturation. (F) The production and secretion of Aβ40 and Aβ42 in pure iNs and iOPCs cultures, measured by commercial ELISA kits (see Table of Materials) on supernatant obtained from pure iNs and iOPCs cultures both at Day 15 and normalized by cell numbers (both at the density of 200,000 cells per well in a 24-well plate). Please click here to view a larger version of this figure.
Data in bar graphs are plotted as mean ± SEM (n ≥ 3). Statistical significance was evaluated by Student’s t-test (*, p < 0.05; ***, p < 0.001); in (D), compared to the NPC; in (E), compared to the control iOPC-Tempo; in (F), compared to iN.
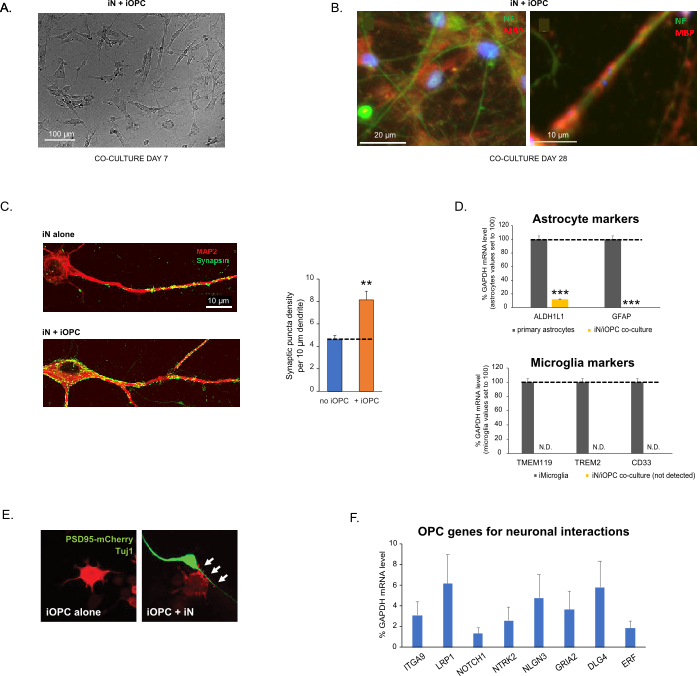
Figure 3: Co-culture of iNs and iOPCs. (A) Representative bright field image of co-cultured iNs and iOPCs at Day 7, showing a proper density for further maturation. (B) Representative immunofluorescence image of iNs and iOPCs co-cultured for 28 days. Axonal marker neurofilament NF is shown in green and oligodendrocytic marker MBP in red. Right, a segment of iN axon ensheathed by iOL process (MBP+). (C) Synapse formation assayed in 4-week-old co-cultures. Cells were stained for Synapsin 1 (Syn1, green) and MAP2 (red), and synaptic puncta were quantified by confocal analysis of density along the dendritic segments as described17,18. (D) In our co-cultures of iNs and iOPCs (7 days of co-culturing), the expression of astrocyte markers, ALDHL1 and GFAP, is minimal (top), and the expression of microglia markers, TMEM119, TREM2, and CD33, is not detected (N.D.) by qPCR. The contamination from these two glial cell types is thus excluded. (E) Coculturing iOPC with iN leads to the formation of neuron-OPC synapses. The fluorescence-tagged post-synaptic marker PSD95-mCherry is expressed only in OPCs, and display a diffuse pattern in single cultures (left) but aggregate to form puncta in cocultures (right, indicated by arrows; Tuj1, neuronal marker). (F) The expression of well-characterized oligodendroglial genes that can sense and respond to neuronal activities in the pure cultures of iOPCs at Day 14. Please click here to view a larger version of this figure.
Data in bar graphs is plotted as mean ± SEM (n ≥ 3). Statistical significance was evaluated by Student’s t-test (**, p < 0.005; ***, p < 0.001); in (C), compared to the no OPC condition; in (D), compared to primary astrocytes in top panel.
Discussion
In addition to the physical and metabolic support to stabilize the synapse structures and to facilitate the saltatory signal conduction by myelination, oligodendrocyte lineage cells can shape neuronal activity pattern via rapid and dynamic cross-talks with neurons5,6,7. While in AD pathology the oligodendroglial responses were initially regarded as merely secondary to inflammation and oxidative stresses, there is now promising evidence arguing that compromised myelin integrity is an early pathogenic event prior to the appearance of Aβ aggregation and tau hyperphosphorylation9. Furthermore, the repair of myelination through self-renewal of OPCs is particularly vulnerable in AD38, a process that heavily depends on neuronal activities. Understanding the mechanism to support healthy neuron-oligodendrocyte signaling thus represent an excellent opportunity for identifying new therapeutic targets.
The single transcription factor Ngn2 protocol is one of the most employed techniques for the generation of stem cell-derived human neurons, and the procedures outlined here are further refinements for obtaining pure neuronal cultures. Our iOPC/iOL protocol has an induction period shorter than the previously published studies (4 to 24 weeks), with a robust yield and purity comparable to other commonly used protocols19,20,22,23,24,25,26,27,28. Our protocol introduces the stepwise differentiation of ES cells to NPCs, OPCs, and finally oligodendrocytes by characterized patterning cues, and generates functional cells that can be used to study the regulation of myelination homeostasis and repair in vitro or in vivo (e.g., by engraftment into the shiverer mouse model) as described in the previous work. The improvement in our protocol is greatly promoted by the DMSO incubation, which activates the retinoblastoma protein and prolongs the G1 phase of the cell cycle to better integrate the stimuli of directed differentiation, and also enhances terminal differentiation into functional derivatives29,30. Finally, the use of clemastine, a muscarinic and antihistaminic compound hit identified through drug screens for remyelination therapeutics33, additionally shortens the oligodendrocyte maturation, as observed in iPS cell preparation and living animals7,28.
The limitations of the technique mainly lie in the intrinsic discrepancy between the simplified in vitro settings and the in vivo milieus in the brain; this discrepancy leads to a discount in full developmental potential at the advanced stages for individual brain cell types. For iNs, recent studies were able to maintain the cultures in good synaptic health for a considerably long period of time, but still revealed some relative immaturity manifested as reduced spine-like structures and impaired spontaneous synaptic transmission in “old” iN cultures (even the 25-month-old ones)39. While iOPCs have been reproducibly shown to myelinate axons in vivo after being transplanted into transgenic mouse brains, the in vitro myelination assays with electron microscopic evaluation still represent a technical challenge with unsatisfactory efficiency for almost all published protocols19,28, including this one. Therefore, our neuron-OPC co-culture system is not anticipated to faithfully mimic the brain-aging process as well as the late stage of AD pathology. Rather, it is uniquely poised to disentangle the elaborate inter-cellular interactions between neurons and OPCs or early-stage oligodendrocyte, which are independent of myelination and yet fundamental for proper neural development and disease pathogenesis.
Each of the three procedures described here has its c ritical steps within the protocol and may require modifications and troubleshooting. For iN protocol, there are two critical steps: puromycin selection (step 1.2.5) and plating density (step 1.2.6). Incomplete removal of under-transduced cell results in the contamination of poorly differentiated cells and compromises the neuronal survival and functions. The modifications for stronger puromycin selection with higher concentration and longer incubation as described in step 1.2.5 would have to be considered. The suitable plating density should be determined by titration for each pluripotent cell line, as low density leads to collapse of the cultures and high density encourages cell aggregation and impedes neuronal growth. For iOPC/iOL protocol, the two critical steps are the control of cell proliferation in OPC differentiation (step 2.2.3) and plating density for OL maturation (steps 2.2.4 and 2.3.2). The overgrowth of differentiating NPCs signals a poor response to the OPC differentiation stimuli and needs to be dampened by an appropriate dosage of Ara-C treatment (within the indicated range). While plating OPCs for maturation, a lower range of cell density is preferred here as the sparse distribution can facilitate the induction of a physiological morphology of complex structures (as shown in Figure 2C). For iN-iOPC co-culturing protocol, we would like to draw attention to the critical step of plating with an appropriate density for both cell types (steps 3.1.1 and 3.2.2). Specifically, the iNs may not attach well to the surface in between growing OPCs and tend to detach first when the culture reaches confluency. The optimal ratio would have to be decided by titrating the cell numbers.
Overall, this reductionist approach residing in our protocols is a powerful tool to dissect the specific hetero-cellular interactions from the inherent complexity of the human brain, and serve to uncover oligodendroglial biology in health and in AD. The significance with respect to existing methods is thus fairly apparent in our opinion. An additional utility of the methods developed here for the future applications is cell-based therapy for demyelinating conditions, such as post-radiotherapy40 and spinal cord injury41,42. Moreover, the high-throughput capacity of this stem cell-based system can also be utilized on a larger scale to screen libraries of small molecules for compounds that can protect or restore the physiological status of neurons, OPCs, oligodendrocytes and their interactions. Thus, we believe the protocols described here will facilitate future work in developing better modeling tools and effective treatments for AD and other neurodegenerative disorders.
Divulgations
The authors have nothing to disclose.
Acknowledgements
This work was supported by the grants from National Institutes of Health (R00 AG054616 to Y.A.H. and T32 GM136566 to K.C.), Stanford University School of Medicine and a Siebel Fellowship (awarded to S.C.). Y.A.H. is a GFL Translational Professor from the Center for Translational Neuroscience in the Brown Institute for Translational Sciences.
Materials
| Accutase | STEMCELL Technologies | 7920 | |
| B27 supplement | ThermoFisher | 17504044 | |
| bFGF | ThermoFisher | PHG 0266 | |
| cAMP | MilliporeSigma | A9501 | |
| Clemastine | MilliporeSigma | SML0445 | |
| DMEM/F12 medium | STEMCELL Technologies | 36254 | |
| DMSO | ThermoFisher | D12345 | |
| Doxycycline | MilliporeSigma | D3072 | |
| Fetal Bovine Serum | ScienCell | 10 | |
| H1 human ES cells | WiCell | WA01 | |
| Matrigel | Corning | 354234 | |
| mTeSR plus | STEMCELL Technologies | 5825 | |
| N2 supplement | ThermoFisher | 17502001 | |
| Neurobasal A medium | ThermoFisher | 10888-022 | |
| Non Essential Amino Acids | ThermoFisher | 11140-050 | |
| PDGF-AA | R&D Systems | 221-AA-010 | |
| PEI | VWR | 71002-812 | |
| pMDLg/pRRE | Addgene | 12251 | |
| Polybrene | MilliporeSigma | TR-1003-G | |
| pRSV-REV | Addgene | 12253 | |
| Puromycin | ThermoFisher | A1113803 | |
| ROCK Inhibitor Y-27632 | STEMCELL Technologies | 72302 | |
| SAG | Tocris | 4366 | |
| STEMdiff Neural Progenitor Freezing Media | STEMCELL Technologies | 5838 | |
| STEMdiff SMADi Neural Induction Kit | STEMCELL Technologies | 8581 | |
| T3 triiodothyronine | MilliporeSigma | T6397 | |
| Tempo-iOlogo: Human iPSC-derived OPCs | Tempo BioScience | SKU102 | |
| TetO-Ng2-Puro | Addgene | 52047 | |
| VSV-G | Addgene | 12259 |
References
- Pelvig, D. P., Pakkenberg, H., Stark, A. K., Pakkenberg, B. Neocortical glial cell numbers in human brains. Neurobiology of Aging. 29 (11), 1754-1762 (2008).
- Barres, B. A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 60 (3), 430-440 (2008).
- De Strooper, B., Karran, E. The cellular phase of Alzheimer’s disease. Cell. 164 (4), 603-615 (2016).
- Monje, M. Myelin plasticity and nervous system function. Annual Review of Neuroscience. 41, 61-76 (2018).
- Hughes, E. G., Orthmann-Murphy, J. L., Langseth, A. J., Bergles, D. E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nature Neuroscience. 21 (5), 696-706 (2018).
- Gibson, E. M., et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 344 (6183), 1252304 (2014).
- Pan, S., Mayoral, S. R., Choi, H. S., Chan, J. R., Kheirbek, M. A. Preservation of a remote fear memory requires new myelin formation. Nature Neuroscience. 23 (4), 487-499 (2020).
- Thornton, M. A., Hughes, E. G. Neuron-oligodendroglia interactions: Activity-dependent regulation of cellular signaling. Neuroscience Letters. 727, 134916 (2020).
- Ettle, B., Schlachetzki, J. C. M., Winkler, J. Oligodendroglia and myelin in neurodegenerative diseases: more than just bystanders. Molecular Neurobiology. 53 (5), 3046-3062 (2016).
- Essayan-Perez, S., Zhou, B., Nabet, A. M., Wernig, M., Huang, Y. A. Modeling Alzheimer’s disease with human iPS cells: advancements, lessons, and applications. Neurobiology of Disease. 130, 104503 (2019).
- Li, L., et al. GFAP mutations in astrocytes impair oligodendrocyte progenitor proliferation and myelination in an hiPSC model of Alexander disease. Cell Stem Cell. 23 (2), 239-251 (2018).
- Lin, Y. T., et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron. 98 (6), 1294 (2018).
- TCW, J., et al. Cholesterol and matrisome pathways dysregulated in human APOE ε4 glia. bioRxiv. , (2019).
- Ang, C. E., Wernig, M. Induced neuronal reprogramming. Journal of Comparitive Neurology. 522 (12), 2877-2886 (2014).
- Penney, J., Ralvenius, W. T., Tsai, L. H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Molecular Psychiatry. 25 (1), 148-167 (2020).
- Zhang, Y., et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 78 (5), 785-798 (2013).
- Huang, Y. A., Zhou, B., Nabet, A. M., Wernig, M., Sudhof, T. C. Differential signaling mediated by ApoE2, ApoE3, and ApoE4 in human neurons parallels Alzheimer’s Disease risk. Journal of Neuroscience. 39 (37), 7408-7427 (2019).
- Huang, Y. A., Zhou, B., Wernig, M., Sudhof, T. C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Abeta Secretion. Cell. 168 (3), 427-441 (2017).
- Yang, N., et al. Generation of oligodendroglial cells by direct lineage conversion. Nature Biotechnology. 31 (5), 434-439 (2013).
- Douvaras, P., et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports. 3 (2), 250-259 (2014).
- Lee, E. H., Park, C. H. Comparison of reprogramming methods for generation of induced-oligodendrocyte precursor cells. Biomolecules & Therapeutics (Seoul). 25 (4), 362-366 (2017).
- Ehrlich, M., et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proceedings of the National Academy of Sciences of the United States of America. 114 (11), 2243-2252 (2017).
- Rodrigues, G. M. C., et al. Defined and scalable differentiation of human oligodendrocyte precursors from pluripotent stem cells in a 3D culture system. Stem Cell Reports. 8 (6), 1770-1783 (2017).
- Hu, B. Y., Du, Z. W., Li, X. J., Ayala, M., Zhang, S. C. Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development. 136 (9), 1443-1452 (2009).
- Izrael, M., et al. Human oligodendrocytes derived from embryonic stem cells: Effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Molecular and Cellular Neuroscience. 34 (3), 310-323 (2007).
- Yamashita, T., et al. Differentiation of oligodendrocyte progenitor cells from dissociated monolayer and feeder-free cultured pluripotent stem cells. PLoS One. 12 (2), 0171947 (2017).
- Wang, S., et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 12 (2), 252-264 (2013).
- Chanoumidou, K., Mozafari, S., Baron-Van Evercooren, A., Kuhlmann, T. Stem cell derived oligodendrocytes to study myelin diseases. Glia. 68 (4), 705-720 (2020).
- Chetty, S., et al. A simple tool to improve pluripotent stem cell differentiation. Nature Methods. 10 (6), 553-556 (2013).
- Li, J., et al. A transient DMSO treatment increases the differentiation potential of human pluripotent stem cells through the Rb family. PLoS One. 13 (12), 0208110 (2018).
- Sambo, D., Li, J., Brickler, T., Chetty, S. Transient treatment of human pluripotent stem cells with DMSO to promote differentiation. Journal of Visualized Experiments: JoVE. (149), (2019).
- Douvaras, P., Fossati, V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nature Protocols. 10 (8), 1143-1154 (2015).
- Mei, F., et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nature Medicine. 20 (8), 954-960 (2014).
- Madhavan, M., et al. Induction of myelinating oligodendrocytes in human cortical spheroids. Nature Methods. 15 (9), 700-706 (2018).
- Zhang, Y., et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 89 (1), 37-53 (2016).
- Grubman, A., et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nature Neuroscience. 22 (12), 2087-2097 (2019).
- Goldman, S. A., Kuypers, N. J. How to make an oligodendrocyte. Development. 142 (23), 3983-3995 (2015).
- Behrendt, G., et al. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia. 61 (2), 273-286 (2013).
- Patzke, C., et al. Neuromodulator signaling bidirectionally controls vesicle numbers in human synapses. Cell. 179 (2), 498-513 (2019).
- Piao, J., et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 16 (2), 198-210 (2015).
- Keirstead, H. S., et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. Journal of Neuroscience. 25 (19), 4694-4705 (2005).
- Kim, D. S., et al. Rapid generation of OPC-like cells from human pluripotent stem cells for treating spinal cord injury. Experimental & Molecular Medicine. 49 (7), 361 (2017).

