Manipulation of Rhythmic Food Intake in Mice Using a Custom-Made Feeding System
Summary
Restricting the timing of food intake has emerged as a promising intervention to attenuate diet-induced metabolic diseases. This manuscript details the construction and use of an efficient system built in-house for measuring and manipulating rhythmic food intake in mice.
Abstract
Rhythmic gene expression is a hallmark of the circadian rhythm and is essential for driving the rhythmicity of biological functions at the appropriate time of day. Studies over the last few decades have shown that rhythmic food intake (i.e., the time at which organisms eat food during the 24 h day), significantly contributes to the rhythmic regulation of gene expression in various organs and tissues throughout the body. The effects of rhythmic food intake on health and physiology have been widely studied ever since and have revealed that restricting food intake for 8 h during the active phase attenuates metabolic diseases arising from a variety of obesogenic diets. These studies often require the use of controlled methods for timing the delivery of food to animals. This manuscript describes the design and use of a low-cost and efficient system, built in-house for measuring daily food consumption as well as manipulating rhythmic food intake in mice. This system entails the use of affordable raw materials to build cages suited for food delivery, following a user-friendly handling procedure. This system can be used efficiently to feed mice on different feeding regimens such as ad libitum, time-restricted, or arrhythmic schedules, and can incorporate a high-fat diet to study its effect on behavior, physiology, and obesity. A description of how wild-type (WT) mice adapt to the different feeding regimens is provided.
Introduction
The circadian clock is found ubiquitously across species and provides a time-keeping mechanism that helps organisms to adapt to their rhythmically changing environment. The master circadian pacemaker is located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN is primarily entrained by the environmental light-dark cycle, and synchronizes peripheral clocks present in nearly every cell of the body via multiple cues, including neuronal and hormonal signals, feeding, and body temperature1,2,3,4,5,6,7,8. In mammals, the molecular circadian clock relies on the heterodimeric transcription factor CLOCK: BMAL19,10, which controls the expression of the core clock genes named Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2) to initiate a transcriptional feedback loop that is critical for the generation of circadian rhythms9,11,12. The molecular clock also regulates the rhythmic transcription of thousands of genes that control the rhythmicity of virtually every biological function13,14,15. More than 50% of the genome in mammals is rhythmically expressed in at least one tissue type16,17,18, and tissues such as the liver in mice have about 25%-30% of their transcriptome expressed rhythmically18,19. Rhythmic gene expression is crucial to activate important biological processes such as cell cycle control20, glucose homeostasis21, and amino acid metabolism22 at the right time of the day in order to increase organism fitness.
Over the past few decades, there has been increasing evidence suggesting that food intake can act as a potent synchronizing cue for entraining rhythms in gene expression in multiple tissues, including the liver23,24. Importantly, feeding has been shown to entrain rhythms in the liver independently of the SCN or of the light-dark cycle25, and rhythmic feeding can drive rhythmic gene expression without involving the molecular clock26,27,28,29,30,31. Feeding restricted to the inactive period of mice (daytime) inverts the phase of expression of the core clock genes and of many rhythmic genes31. Time-restricted feeding (TRF), which is a nutritional intervention where the daily caloric intake is restricted to a period of 8-10 h, has been shown to protect against obesity, hyperinsulinemia, hepatic steatosis, and metabolic syndrome32,33. All the above experiments involving manipulation of food intake require the experimenter to make use of effective methods to deliver food at the right time of day.
Different methods of food delivery have been developed, bearing several advantages and disadvantages29,34,35,36,37,38,39 (Table 1). Some automated feeders have been designed to operate based on a software that controls the amount, duration, and timing of food availability while recording feeding and voluntary wheel-running activity in mice34. A few other methods involve mice being placed in different cages for different feeding conditions, with the experimenter manually adding food pellets at the prerequisite time38,39. Another system uses an automated feeder system controlled by a computer where a pneumatic-driven shield prevents access to food and which can be controlled either by time intervals or mass of food35. All these methods either require utilization and setup of a computerized software that can be expensive and require some training for proper operation of the instrument or are labor intensive because the experimenter needs to be present at specific times to manually change feeding conditions. Computerized systems also come with their share of issues, including malfunctioning of levers or doors that let the food out, food pellets getting stuck in the outlets, and software breakdown. Moreover, the sound that may be produced during the opening of doors or levers presents the risk of conditioning mice to associate these with food delivery, thereby compromising the interpretation of effects of food manipulation as being strictly due to food access or due to effects on other behavioral rhythms such as sleep/wake cycle. The overall goal of this study was to develop an affordable and efficient system to manipulate long-term rhythmic food intake that would help alleviate many of these aforementioned issues. First and foremost, the feeding apparatus that was developed and is described below can be constructed at a very minimal cost compared to the automated machines (Table 2) and does not require sophisticated training for handling, operation, and maintenance. Secondly, the feeding system only produces a background white noise and no loud sounds during food delivery, thereby preventing Pavlovian conditioning. Altogether, this feeding system is economic, more accessible, and reliable for researchers while still being efficient in the manipulation of rhythmic food intake.
Protocol
All the animal subjects are used in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of Texas A&M University (AUP #2022-0050). Both male and female C57BL/6 mice between 2-4 months of age are used here. The procedure for building the feeding system is described below, and the raw materials required to build the apparatus are referenced in the Table of Materials.
1. Construction of the feeding system
- Constructing a poly vinyl chloride (PVC) base
- Procure four pieces of 0.25-inch PVC sheets cut as per the following dimensions: 4.875 inch x 4.5 inch; 4.875 inch x 2.125 inch; 9.5 inch x 2.125 inch (two pieces). Drill four holes in the 4.875-inch x 4.5-inch base for attaching a 24 h timer using the measurements provided in Figure 1A. Glue the four pieces of PVC to obtain the base as in Figure 1A.
- Securing the timer on the PVC base
- Open the timer to remove the plug. Make a connection using a standard extension/electric cord (Figure 1B).
- Place the timer on the PVC base to align it with the holes drilled on the base in step 1.1.1. Use 1.5-inch screws to fix the timer on the PVC base. Make sure the timer lies flat and is stable on the PVC base.
- Drill four holes on top of the timer and fix 0.75-inch screws to hold the eight-compartment food container (Figure 1B). The base for the system is complete and looks like Figure 1C.
CAUTION: Do not drill the holes through the entire timer.
- Cage setup
- Cut a 4-inch PVC tube (external diameter of 4.5 inch) at a 3.125-inch height. Drill a hole of about 0.5 inch at the bottom of the pipe to let the electric cable through.
- Sand the top of the pipe (e.g., with a router or a rotary tool) to allow the cap to be removed easily when changing the food container.
- Use a mouse cage having a width greater than 4.5 inch and cut a 4.5-inch hole at the bottom of the cage using a hole saw.
NOTE: The location of the hole depends on the cage setup (e.g., location of the cage top and water bottle, addition of a running wheel, etc.).
- Feeding container setup
- Make a food dispenser out of a 4-inch eight-compartment jewelry organizer as shown in Figure 1D. Cut the rim of the container such that it fits well into the pipe.
- Use a 4-inch PVC pipe cap and cut a hole corresponding to the size of a single dispenser compartment (e.g., with a rotary tool) to create an opening that exposes only one of the eight compartments at a time. As the timer moves, the opening exposes a new compartment every 3 h. The feeding system components are now ready to be used. Once all the cages are set, the final setup resembles the one shown in Figure 1E.
- Transport of many food containers at a time can be cumbersome. To facilitate easier transport, take three pieces of 0.25-inch PVC pipe. Drill a 0.625-inch hole in the center of two pieces and glue them together. Then, use a narrow 0.625-inch PVC pipe that fits in the hole and through the center of the food cups to stack food cups to facilitate their transport, as shown in Figure 1F.
- Test the timers prior to introducing the mice by plugging the setup into power outlets, placing a piece of nestlet in one compartment at a recorded time, and monitoring the position of the nestlet 12 h later to ensure that the timer turns promptly.
2. Application of the feeding system
- Continuous measurement of food intake in mice
- Transfer mice to the experimental room and acclimate them to the light-dark (LD) cycle set in the room for at least 1 week, and for 2 weeks if the light-dark cycle is shifted by more than 3 h. For this experiment, data is collected from male and female C57BL/6 mice between 2-4 months of age exposed to LD 12:12 (n = 7 males and 4 females).
- Record mice weight prior to individually housing them in the feeder cages (weights were recorded at 3:00 pm, i.e., at the time food changeouts were carried out). Make sure that mice have ad libitum access to water and enough bedding and nestlets.
- Add 1.5 g of food (routinely used 45 mg of dustless precision pellets) to all eight compartments of the feeder cup. Place the feeder cup on the timer. Then, put the lid on the feeder cup such that only one compartment is exposed, and note the time of food presentation. Four compartments represent nighttime points and the other four represent day time points.
NOTE: Due to hoarding behavior observed upon excess food presentation, 1.5 g was optimized as the starting food weight. Mice tend to hoard pellets either on top of food cup lids or in the bedding within the cage. This skews the data leading to its misinterpretation. Male mice do not hoard pellets when fed with 1.5 g or less per compartment. Females tend to hoard food more than males, but this is mouse-specific and can be attenuated if providing 1 g of pellet per compartment. - Change food every day at the same time and count the number of pellets remaining in each compartment to calculate the amount of food consumed. Monitor the feeding profile for a week to get a baseline feeding profile of mice fed ad libitum.
- Based on the food consumed in every compartment, calculate the average daily food consumption for each mouse (Figure 2).
- High fat diet (HFD) treatment
NOTE: This feeding system can also be used to study the effect of HFD on metabolic diseases and eventually be used for time-restricted feeding schedules. HFD is not commercially available as precise size and weight pellets, and the pellets for feeding are usually procured as 0.5-inch pellets.- Place the HFD pellets on a clean surface or transparent film and cut them into 6-7 smaller pieces of even size using a razor blade. Cut the pellets small enough so that they resemble normal chow pellets as used in section 2.1 above.
NOTE: Mice tend to hoard bigger pellets in their cages, leading to miscalculation of food consumed. - Weigh out 1.5 g of cut HFD pieces and place in each of the 8 food compartments. For food changeout every other day, 1.5 g of HFD per compartment is sufficient.
- Change the food every day or every other day as per experimental needs and record the weight of the food that remains.
- Calculate the amount of food consumed by subtracting the remaining weight of food from the initial amount of food given. Repeat this process over a period of 1 week to obtain a baseline of HFD food intake (Figure 2).
- Place the HFD pellets on a clean surface or transparent film and cut them into 6-7 smaller pieces of even size using a razor blade. Cut the pellets small enough so that they resemble normal chow pellets as used in section 2.1 above.
- Acclimating male mice to a night restricted (NR) diet
- Follow steps 2.1.1-2.1.4 to obtain a baseline of ad libitum feeding. For this experiment, data is collected from male C57BL/6 mice between 2-4 months of age exposed to LD 12:12 (n = 18 males).
- After 3-7 days of ad libitum diet, put the mice on a transition diet by gradually reducing the number of pellets in the day compartments. To do this, have five pellets per compartment on day 1 of transition (0.225 g per compartment), 3 pellets on day 2 (0.135 g per compartment), 1 pellet on day 3 (0.045 g per compartment), and none thereafter to completely transition the mice on a night restricted diet.
NOTE: Ensure that mice are not calorie restricted. Average the daily food consumption of mice per compartment based on the ad libitum baseline and give them the same amount of food, distributing it only across the four-night compartments. - Keep monitoring the food intake for 2 weeks after the mice have adapted to the night restricted regimen. During this period, adjust the amount of food given to each mouse to better suit its total food consumption (Figure 3A). Typically add food pellets (1 pellet for each of the four night compartments) when mice eat all their food for two consecutive nights.
- Weigh the mice at the end of the 2-week period to monitor any change of weight due to the feeding regimen. At the end of this period, anesthetize the mice with isoflurane and euthanize them by decapitation. Collect tissues and analyze them for daily changes due to the feeding paradigm.
- Acclimating male mice to an arrhythmic (AR) diet
- Follow steps 2.1.1-2.1.4 to obtain a baseline of ad libitum feeding. For this experiment, data is collected from male C57BL/6 mice between 2-4 months of age exposed to LD 12:12 (n = 18 males).
- After a week of ad libitum diet, calculate the average food consumption per day and divide that number by 8 to obtain the amount of food to be provided in each compartment. Achieve AR feeding by ensuring that mice get an equal amount of food in all eight compartments throughout the day.
- Then, put the mice on a transition diet by gradually reducing, over 3-5 days, the amount of food given per compartment to ultimately abolish any rhythm of food intake (as shown in Figure 3B). When on an AR diet, ensure that the mice have access to 1/8 of their daily food intake in each of the eight compartments, and thus food access at every 3 h. Ensure that mice are not calorie restricted.
- Maintain the mice on AR diet for 2 weeks or more (Figure 3B).
- During AR diet, adjust the food every day to make sure that mice leave only a few pellets behind (typically less than 5). This ensures that mice are getting just the right amount of food and are not calorie restricted. Make adjustments by reducing or adding pellets in either all the eight compartments or by reducing or adding pellets in two opposite compartments, to not induce any rhythms of food intake.
NOTE: Mice fed under an AR diet leave food almost exclusively between ZT3 and ZT9 (between 3 h and 9 h after light on) yet are hungry at night and bite the food dispenser to access the next compartment. Nevertheless, AR fed mice are not calorie restricted, and in fact gain more weight than NR fed mice over time. - Weigh the mice at the end of the 2-week period to monitor any change of weight due to the feeding regimen. At the end of this period, anesthetize the mice with isoflurane and euthanize them by decapitation. Collect tissues and analyze them for daily changes due to the feeding paradigm.
Representative Results
The feeding system described above can be used for long term manipulation of rhythmic food intake in mice. This system essentially exposes a new food compartment to the mouse every 3 h enabling the researcher to specifically manipulate food in every compartment. One application was to analyze the profile of food intake over the 24 h period. The data indicate that WT mice fed normal chow ad libitum eat about 75% of their food during the night (Figure 2A). Moreover, most of the food eaten during the day occurs within the 3 h before light off.
Mice fed HFD ad libitum ate more food on the first 2 days of exposure, likely because of the novelty of HFD (Figure 2A). After 2 days, the HFD intake remained rhythmic, yet with a decreased amplitude compared to when fed normal chow ad libitum. While both male and female WT mice were fed HFD, it was found that female mice hoarded a large amount of food on the lid of the feeding apparatus and in the cage, whereas males did not show any noticeable hoarding. As mentioned above, hoarding food can result in miscalculating food consumption and lead to data misinterpretation. In addition, female mice bit into the plastic rims of the food cups more frequently, especially in the night compartments. Male mice showed significant weight gain after 1 week of ad libitum normal chow and after 1 week of HFD (Figure 2E). A similar trend was observed with female mice but did not reach significant p-values, likely in part because of the lower number of females used compared to males.
Mice transitioned to a NR diet eat their daily total calories only at night, without a significant decrease in calorie intake for the first 3-5 weeks (Figure 3A). Longer exposure to NR schedule decreases the daily average calorie intake by 10%-15% compared to mice fed ad libitum, as described elsewhere34. Mice transitioned to an AR diet consumed their daily total calories in equal quantities across the day, leading to a dramatic dampening of the daily rhythm of food intake (Figure 3B). As for the NR feeding schedule, the daily average of calorie intake is not affected by the AR feeding schedule for the first 3-5 weeks of exposure but decreases with longer exposure. Mice showed weight gain after the NR (Figure 3C) and AR schedules (Figure 3C).
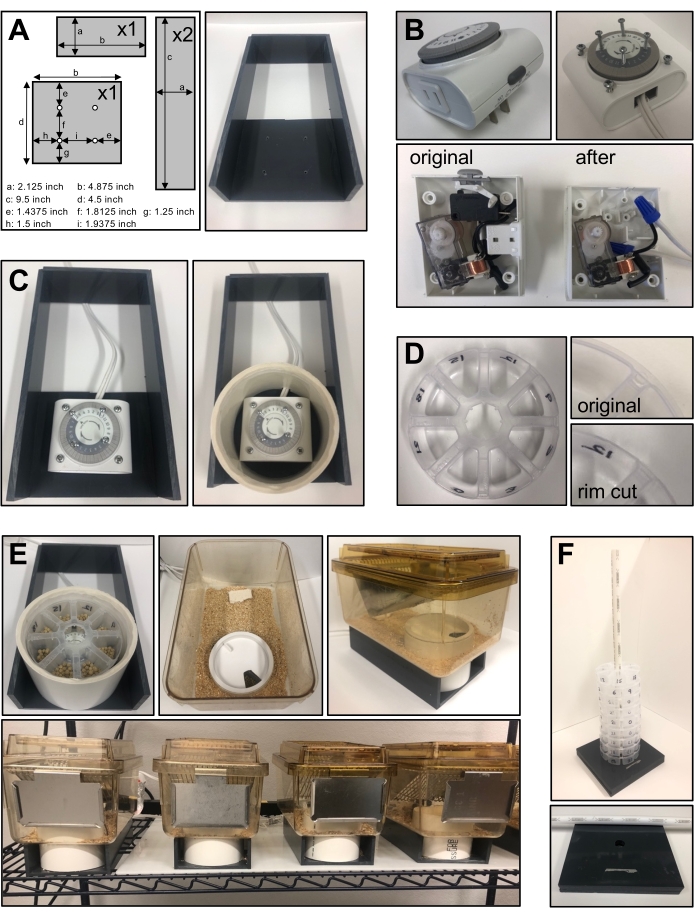
Figure 1: Design and construction of the feeding apparatus system. (A) The dimensions of the PVC base for the feeding system, and description of where holes need to be made for fixing the timer. (B) A 24 h timer before and after repurposing the wire and drilling with screws for placement of the food cup. (C) The assembled gray base and timer along with a 4-inch PVC pipe. (D) The eight-compartment food cup after trimming outer edges. (E) The final setup of cages with the food cup covered with a 4-inch cap such that only one compartment is accessible at a time. (F) The transportation of multiple food cups during an experiment. Please click here to view a larger version of this figure.
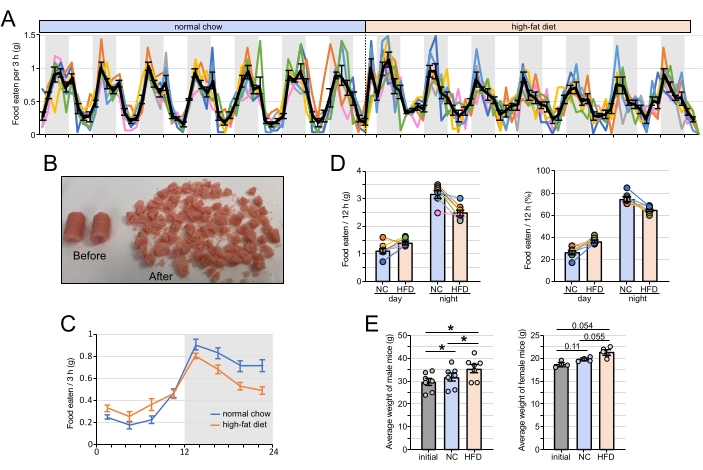
Figure 2: Feeding profiles under different dietary regimes. (A) The feeding profile of male WT mice fed ad libitum with normal chow (NC) for 7 days and high-fat diet (HFD) for an additional 7 days. Colored lines represent individual mouse profiles (n = 7) and the black line indicates the average ± SEM of seven mice. (B) The high-fat diet before and after slicing. (C) The daily average of food intake every 3 h ± SEM (n = 7). The average was calculated over the last 5 days of either the NC or the HFD feeding schedule. (D) The average (left) and the percentage (right) of food intake during the day and night for mice fed with NC or HFD. The values represent the average of seven mice ± SEM and were calculated using the food intake data over the last 5 days of either NC or HFD feeding schedules. * p < 0.05 between the two groups (paired t-test). (E) The average body weights of mice used in the experiment after 1 week of NC and 1 week of HFD. The data for males (left) and females (right) is shown with * p < 0.05 between the two groups (paired t-test). Please click here to view a larger version of this figure.
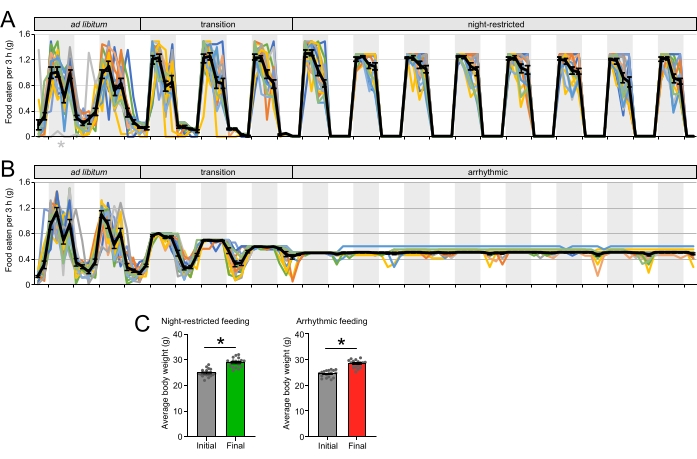
Figure 3: Manipulation of the daily rhythm of food intake. (A) The feeding profile of male WT mice fed with normal chow ad libitum for 2 days, transitioned to a night-restricted (NR) feeding regime for 3 days, and maintained under NR feeding for 8 nights. The colored lines represent individual mouse profiles (n = 18) and the black line indicates the average ± SEM of 18 mice. The gray asterisk indicates malfunctioning of the timer for that mouse on that single day where the timer stopped turning. (B) Feeding profile of male WT mice fed with normal chow ad libitum for 2 days, transitioned to an arrhythmic (AR) feeding regime for 1 day, and maintained under AR feeding for 8 nights. The colored lines represent individual mouse profiles (n = 18) and the black line indicates the average ± SEM of the 18 mice. (C) The average body weights of mice used in the experiment after 2 weeks of exposure to NR and AR diets. Data is shown with * p < 0.05 between the two groups (paired t-test). Please click here to view a larger version of this figure.
Table 1: Advantages and disadvantages of the existing feeding systems. A table highlighting different feeding systems used for manipulation of food intake, with a brief description of the pros and cons of each system. Please click here to download this Table.
Table 2: Cost of materials required to construct the feeding system. A table listing the cost of items required for the construction of the feeding system described in this paper along with an estimate of the cost of construction per cage. Please click here to download this Table.
Discussion
Extensive research has been carried out over the last few decades on the manipulation of feeding rhythms and their effect on physiology. The construction and utilization of the feeding system described here can be used as an efficient method for manipulating food intake. The protocol utilizes a common 24 h timer and a food cup designed as an eight-compartment organizer as key components of the system. The cages can be constructed with ease using just a few easily accessible tools, and the handling of the system is user-friendly. Some of the key aspects of the protocol for adapting the system to manipulate rhythmic food intake include daily changing of food cups since the timer turns across a 24 h period, manual counting or weighing of remaining food, and daily adjustment of the number of pellets for AR feeding. Typically, plastic shavings are seen when mice are hungry and do not get adequate food. This issue can be fixed by adding a few more food pellets abiding by the feeding regimen until no plastic shavings are seen. In the case of AR feeding, where daily food needs to be adjusted, care should be taken not to induce the rhythm of food intake (Figure 3B). Hence, it is preferable to either add or subtract pellets in opposite compartments throughout to keep mice arrhythmically fed.
This system can be further improved by coating the food cups with a layer of epoxy to prevent the mice from biting the plastic, and thus help prolong the life of the food cups. The surface of the timer for food cup placement can also be modified to help the food cup sit flat and stable on the timer. This could prevent the accidental stopping of the timer caused by an unevenly placed timer. A few of the cage components, such as food cups, can also be 3D printed to reduce cost and custom-made to the liking of the researcher. This can include food cups with more than eight compartments, which can give a better time resolution than the current 3 h window.
Although very efficient, this system has some limitations, such as being labor intensive, with the researcher still needing to change food cups every 24 h and requiring them to manually count/weigh the remaining food. In addition, the timers need to be monitored from time to time to identify potential issues and/or if they stopped working. This can be achieved while counting the food pellets remaining after feeding (e.g., by determining whether some mice ate food only in a few compartments and left some compartments untouched). Another limitation of this system is that it may not work as well with female mice, since the few experiments performed with females showed that they tend to hoard food and chew the plastic more than male mice.
Nevertheless, this feeding system is very effective in manipulating food intake, is easy to construct, operate, maintain, and is inexpensive compared to the expensive automated feeders existing in the market. It can be easily adapted and modified to suit the researcher’s requirements and does not need any special training to operate the system. Importantly, timers only produce a low amount of constant white noise, which prevents mice from associating any sound with food availability.
In summary, this paper describes an innovative feeding system that can be used to monitor daily food consumption in mice and can be adapted to feed mice on different paradigms such as time-restricted feeding, arrhythmic feeding, and feeding on high-fat diet. This system adds to the list of tools that can be used to address important questions in the field of rhythmic food intake and its effect on physiology.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was financially supported by the grant R01DK128133 from NIH/NIDDK (to J.S.M) and startup funds from Texas A&M University.
Materials
| #6 x 0.75 inch Phillips Pan Head Stainless Steel Sheet Metal Screw (50-Pack) | Everbilt | #800172 | |
| #8 x 1.5 inch Phillips Pan Head Zinc Plated Sheet Metal Screw (100-Pack) | Everbilt | #801622 | |
| 0.25 inch gray PVC sheet (24 inch x 48 inch) | USPlastic | #45088 | |
| 4 inch PVC pipe (10 ft) | Home Depot | #531103 | |
| 45 mg dustless precision pellets | Bio-Serv | #F0165 | |
| 6 ft. Extension Cord | HDX | HD#145-017 | |
| Food container (eight-compartment jewelry organizer) | JewelrySupply | #PB8301 | |
| Indoor Basic Timer | General Electric | #15119 | |
| Oatey 4 inch ABS Pipe Test Cap with Knockout | Home Depot | #39103D | |
| Rodent Diet with 45 kcal% fat (with red dye) | Research Diets | #D12451 |
Referencias
- Boothroyd, C. E., Wijnen, H., Naef, F., Saez, L., Young, M. W. Integration of light and temperature in the regulation of circadian gene expression in Drosophila. PLoS Genetics. 3 (4), 0492-0507 (2007).
- Brown, S. A., Zumbrunn, G., Fleury-Olela, F., Preitner, N., Schibler, U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Current Biology. 12 (18), 1574-1583 (2002).
- Buhr, E. D., Yoo, S. H., Takahashi, J. S. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 330 (6002), 379-385 (2010).
- Kawamoto, T., et al. Effects of fasting and re-feeding on the expression of Dec, Per1, and other clock-related genes. Journal of Biochemistry. 140 (3), 401-408 (2006).
- Lamia, K. A., Storch, K. F., Weitz, C. J. Physiological significance of a peripheral tissue circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 105 (39), 15172-15177 (2008).
- Oosterman, J. E., Kalsbeek, A., La Fleur, S. E., Belsham, D. D. Impact of nutrients on circadian rhythmicity. American Journal of Physiology – Regulatory Integrative and Comparative Physiology. 308 (5), 337-350 (2015).
- Pitts, S. N., Perone, E., Silver, R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. American Journal of Physiology – Regulatory Integrative and Comparative Physiology. 285, 57-67 (2003).
- Sheward, W. J., et al. Entrainment to feeding but not to light: Circadian phenotype of VPAC 2 receptor-null mice. Journal of Neuroscience. 27 (16), 4351-4358 (2007).
- Gekakis, N., et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 280 (5369), 1564-1569 (1998).
- King, D. P., et al. Positional cloning of the mouse circadian Clock gene. Cell. 89 (4), 641-653 (1997).
- Kume, K., et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 98 (2), 193-205 (1999).
- Shearman, L. P., et al. Interacting molecular loops in the mammalian circadian clock. Science. 288 (5468), 1013-1019 (2000).
- Beytebiere, J. R., et al. Tissue-specific BMAL1 cistromes reveal that rhythmic transcription is associated with rhythmic enhancer-enhancer interactions. Genes and Development. 33 (5-6), 294-309 (2019).
- Menet, J. S., Pescatore, S., Rosbash, M. CLOCK: BMAL1 is a pioneer- like transcription factor. Genes and Development. 28 (1), 8-13 (2014).
- Koike, N., et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 338 (6105), 349-354 (2012).
- Mure, L. S., et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 359 (6381), (2018).
- Ruben, M. D., et al. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Science Translational Medicine. 10 (458), 1-8 (2018).
- Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E., Hogenesch, J. B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proceedings of the National Academy of Sciences of the United States of America. 111 (45), 16219-16224 (2014).
- Menet, J. S., Rodriguez, J., Abruzzi, K. C., Rosbash, M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife. 2012 (1), 1-25 (2012).
- Miller, B. H., et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 104 (9), 3342-3347 (2007).
- Cailotto, C., et al. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: Are the clock genes involved. European Journal of Neuroscience. 22 (10), 2531-2540 (2005).
- Eckel-Mahan, K. L., et al. Coordination of the transcriptome and metabolome by the circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 109 (14), 5541-5546 (2012).
- Damiola, F., et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes and Development. 14 (23), 2950-2961 (2000).
- Saini, C., et al. Real-time recording of circadian liver gene expression in freely moving mice reveals the phase-setting behavior of hepatocyte clocks. Genes and Development. 27 (13), 1526-1536 (2013).
- Stokkan, K. A., Yamazaki, S., Tei, H., Sakaki, Y., Menaker, M. Entrainment of the circadian clock in the liver by feeding. Science. 291 (5503), 490-493 (2001).
- Atger, F., et al. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proceedings of the National Academy of Sciences of the United States of America. 112 (47), 6579-6588 (2015).
- Greenwell, B. J., et al. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice. Cell Reports. 27 (3), 649-657 (2019).
- Izumo, M., et al. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. eLife. 3, 04617 (2014).
- Mange, F., et al. Diurnal regulation of RNA polymerase III transcription is under the control of both the feeding-fasting response and the circadian clock. Genome Research. 27 (6), 973-984 (2017).
- Van Der Veen, D. R., et al. Impact of behavior on central and peripheral circadian clocks in the common vole Microtus arvalis, a mammal with ultradian rhythms. Proceedings of the National Academy of Sciences of the United States of America. 103 (9), 3393-3398 (2006).
- Vollmers, C., et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proceedings of the National Academy of Sciences of the United States of America. 106 (50), 21453-21458 (2009).
- Chaix, A., Lin, T., Le, H. D., Chang, M. W., Panda, S. Time-Restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metabolism. 29 (2), 303-319 (2019).
- Hatori, M., et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism. 15 (6), 848-860 (2012).
- Acosta-Rodríguez, V. A., de Groot, M. H. M., Rijo-Ferreira, F., Green, C. B., Takahashi, J. S. Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metabolism. 26 (1), 267-277 (2017).
- Chung, H., et al. Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism: Clinical and Experimental. 65 (12), 1743-1754 (2016).
- Sen, S., et al. Ultradian feeding in mice not only affects the peripheral clock in the liver, but also the master clock in the brain. Chronobiology International. 34 (1), 17-36 (2017).
- Xie, X., et al. Natural food intake patterns do not synchronize peripheral clocks. BMC Biology. 18 (160), 1-11 (2020).
- Swamy, S., et al. Circadian disruption of food availability significantly reduces reproductive success in mice. Hormones and Behavior. 105, 177-184 (2018).
- Xin, H., et al. Protocol for setup and circadian analysis of inverted feeding in mice. STAR Protocols. 2 (3), 100701 (2021).

