Evaluation of the Spindle Assembly Checkpoint Integrity in Mouse Oocytes
Summary
Error in chromosome segregation is a common feature in oocytes. Therefore, studying the spindle assembly checkpoint gives important clues about the mechanisms needed to produce healthy eggs. The present protocol describes three complementary assays to evaluate spindle assembly checkpoint integrity in mouse oocytes.
Abstract
Aneuploidy is the leading genetic abnormality causing early miscarriage and pregnancy failure in humans. Most errors in chromosome segregation that give rise to aneuploidy occur during meiosis in oocytes, but why oocyte meiosis is error-prone is still not fully understood. During cell division, cells prevent errors in chromosome segregation by activating the spindle assembly checkpoint (SAC). This control mechanism relies on detecting kinetochore (KT)-microtubule (MT) attachments and sensing tension generated by spindle fibers. When KTs are unattached, the SAC is activated and prevents cell-cycle progression. The SAC is activated first by MPS1 kinase, which triggers the recruitment and formation of the mitotic checkpoint complex (MCC), composed of MAD1, MAD2, BUB3, and BUBR1. Then, the MCC diffuses into the cytoplasm and sequesters CDC20, an anaphase-promoting complex/cyclosome (APC/C) activator. Once KTs become attached to microtubules and chromosomes are aligned at the metaphase plate, the SAC is silenced, CDC20 is released, and the APC/C is activated, triggering the degradation of Cyclin B and Securin, thereby allowing anaphase onset. Compared to somatic cells, the SAC in oocytes is not as effective because cells can undergo anaphase despite having unattached KTs. Understanding why the SAC is more permissive and if this permissiveness is one of the causes of chromosome segregation errors in oocytes still needs further investigation. The present protocol describes the three techniques to comprehensively evaluate SAC integrity in mouse oocytes. These techniques include using nocodazole to depolymerize MTs to evaluate the SAC response, tracking SAC silencing by following the kinetics of Securin destruction, and evaluating the recruitment of MAD2 to KTs by immunofluorescence. Together these techniques probe mechanisms needed to produce healthy eggs by providing a complete evaluation of SAC integrity.
Introduction
Aneuploidy, which arises from errors in chromosome segregation, is the leading cause of early miscarriages and is highly linked to mistakes in meiosis1. Meiosis is distinct from mitosis because it consists of two rounds of cell division without an intervening DNA replication step. In meiosis I, homologous chromosomes separate while sister chromatids remain together. In oocytes, this step is error-prone, leading to aneuploid egg production2.
To prevent chromosome segregations errors, most cell types activate a surveillance mechanism that pauses the cell cycle, called the spindle assembly checkpoint (SAC). This mechanism senses kinetochore (KT)-microtubule (MT) attachments and the tension is generated when chromosomes are oriented in a bipolar manner3. Unattached kinetochores trigger a SAC response which starts with the recruitment of MPS1, the master regulator of the SAC, to kinetochores3,4. MPS1 initiates the recruitment of other SAC components, acting as a platform to form the mitotic checkpoint complex (MCC). The MCC, composed of MAD1, MAD2, BUB3, and BUBR1, diffuses into the cytoplasm and inhibits APC/C activation by sequestrating its activator CDC20. Once all kinetochores are stably attached to MTs and chromosomes are aligned at the metaphase plate, the SAC is silenced, and the MCC disassembles and releases CDC20, thereby allowing APC/C activation. Active APC/C degrades Securin and Cyclin B, two key steps in triggering anaphase onset5,6. In somatic cells, the SAC is stringent because it is activated by a single unattached kinetochore and is sufficient to induce cell-cycle arrest6. However, during oocyte meiosis, the SAC is more permissive, and oocytes can enter anaphase I with one or more unattached kinetochores6,7,8,9,10. Understanding why the SAC is more permissive in oocytes is an ongoing area of focus in the field. Mechanisms that cause defects in SAC activation or SAC silencing could lead to errors in chromosome segregation or prolonged cell cycle arrest and cell death. Therefore, evaluating the mechanisms that maintain SAC integrity in oocytes is important to understanding the process of forming healthy, euploid eggs.
This protocol describes techniques to comprehensively evaluate the SAC integrity in mouse oocyte meiosis by examining different critical steps of the checkpoint. First, the evaluation of the SAC response after inducing SAC activation is described. This activation is achieved by generating unattached kinetochores using nocodazole, a drug that depolymerizes MTs11. Second, a method to monitor SAC silencing is described by tracking the dynamics of Securin degradation during oocyte maturation. Finally, an immunofluorescence-based assay is employed to measure the recruitment of MAD2, one of the MCC components, to kinetochores. Together, these assays comprehensively assess SAC integrity during oocyte meiotic maturation.
Protocol
All mice used in these protocols were housed and raised according to the Rutgers University Institutional Animal Use and Care Committee (Protocol 201702497) and National Institutes of Health guidelines. These regulatory bodies approved all experimental procedures involving animal studies. All mice used in the present study were 6-8-week-old CF-1 females.
1. Experimental preparation
- Before starting the SAC evaluations, collect mouse oocytes following the previously published report12. Divide collected oocytes into three evenly sized groups and keep them in Chatot, Ziomek, and Bavister (CZB) culture media containing 2.5 µM milrinone (see Table of Materials) to avoid meiotic resumption13.
NOTE: CZB composition: 81.6 mM NaCL; 4.8 mM KCl; 1.2 mM KH2PO4; 1.2 mM MgSO4-7H2O; 0.27 mM Pyruvate; 1.7 mM CaCl2; 30.8 mM DL-Lactic Acid; 7 mM Taurine; 0.1 mM EDTA; 25 mM NaHCO3; Gentamicin; and 0.3% BSA. - Prepare the cRNA for microinjection as described previously12,14. Amplify the mouse Securin gene from the cDNA cloned from mouse germinal vesicle oocytes, followed by subcloning into the pMDL2 vector containing Gfp sequence15,16.
- To prepare Securin-Gfp cRNA, linearize the plasmid with Nde I digestion. Perform in vitro transcription by using T3 RNA polymerase and purifying the cRNA16.
NOTE: Store cRNA in aliquots of 2-3 µL at -80 °C until use.
- To prepare Securin-Gfp cRNA, linearize the plasmid with Nde I digestion. Perform in vitro transcription by using T3 RNA polymerase and purifying the cRNA16.
2. Nocodazole treatment and live imaging
- Prepare 1 mL of culture media (CZB) containing 5 µM nocodazole (NOC), 1 mL of culture media with 5 µM of NOC plus 0.5 µM reversine (NOC + REV), and 1 mL of culture media with Dimethyl sulfoxide (DMSO) (1:2000) as a control (see Table of Materials).
- For oocyte maturation and live imaging, use a 96-well plate pre-warmed in the incubator at 37 °C, 5% CO2, and 80% humidity. In the first well, load 150 µL of the control DMSO treatment; in the second well, load 150 µL of NOC treatment; and in the third well, load 150 µL of NOC + REV treatment. Keep the plate in the incubator under the same conditions as above until needed.
NOTE: In the 96-well plate, avoid using the first row and the first column because the shadow of the border interferes with the quality of the images. - To start meiotic maturation, remove milrinone. While viewing the oocytes under a stereomicroscope using magnification between 30x-64x, wash milrinone out of the media by sequentially transferring the oocytes through six drops of 100 µL of milrinone-free culture media containing DMSO and place them into the corresponding well of the 96-well plate using a hand- or mouth-operated pipette.
- Count oocytes by picking them up using a hand- or mouth-operated pipette and delivering them to the next drop to avoid losing any. The oocytes are single, large (~80 µm in diameter), and round cells. The nucleus will appear like a button in the center of the cell, and the membrane and zona pellucida surround the oocyte.
NOTE: Transfer the oocytes among drops while moving the least amount of liquid possible. This allows for optimal removal of the milrinone from the culture media. If oocytes fail to resume meiosis, it is likely that the milrinone was not effectively removed.
- Count oocytes by picking them up using a hand- or mouth-operated pipette and delivering them to the next drop to avoid losing any. The oocytes are single, large (~80 µm in diameter), and round cells. The nucleus will appear like a button in the center of the cell, and the membrane and zona pellucida surround the oocyte.
- Repeat the same process (step 2.3) for NOC and NOC + REV treatment.
- Image the oocytes using a brightfield microscope equipped with an incubator chamber with a controlled environment under these conditions: 37 °C, 5% CO2, and 80% humidity. Capture images at the middle plane of the oocytes at intervals of 20 min for 24 h.
- Quantify the number of oocytes that extrude a polar body (PB) by identifying the cells that go through asymmetrical cytokinesis. The result will be a small cell (PB) next to the egg and within the shared zona pellucida. View the images using imaging software (ImageJ, see Table of Materials).
- Import image sequence into the image analysis software and advance the frames until one or more cells go through asymmetrical cytokinesis. Calculate the percentage of oocytes that extrude a PB of the total of oocytes17.
3. Monitoring the pattern of Securin-gfp degradation during meiotic maturation
- Centrifuge 3 µL of Securin-Gfp cRNA(step 1.2) at 19,283 x g for 30 min at 4 °C18.
NOTE: Use the supernatant to avoid loading impurities that could cause needle blockage during microinjection. - Follow step-by-step oocyte microinjection, extensively described in reference12. Microinject prophase I-arrested oocytes with 100 ng/µL of Securin-gfp cRNA prepared in step 1.2.
- After microinjection, allow the oocytes to recover and translate the RNA in the CO2 incubator for at least 3 h. Check the expression by observing the oocytes in an automated multichannel fluorescence imaging system (see Table of Materials) to view the GFP signal.
- As described in step 2.2, load 150 µL of culture media with or without 5 µM of nocodazole and 150 µL of culture media with 0.5 µM of reversine in three different wells of a 96-well plate.
- Wash the microinjected oocytes through six drops of milrinone-free culture media and transfer 1/3 of the oocytes into each treatment.
- Keep the plate in the incubator at 37 °C, with 5% CO2 and 80% humidity until the oocytes resume meiosis, around 3 h post milrinone washing.
NOTE: Use a stereomicroscope with magnification between 30x-64x to evaluate the nuclear envelope breakdown as a hallmark of meiotic resumption. - Record images of oocyte maturation using a fluorescence microscope equipped with an incubator chamber as described in step 2.6. Use brightfield settings to find the oocytes in the well. Use a 488 filter to detect the Securin-gfp signal and adjust the fluorescence intensity to avoid over-exposing the cells.
- If the imaging system is automated, save the positions of the oocytes in each well. Because Securin-gfp is evenly distributed into the cytoplasm, capture images in the middle plane of the oocytes at intervals of 20 min for 24 h. To capture groups of oocytes in one picture, use a lower magnification objective lens such as 10x.
- Use image analysis software to quantify Securin-gfp destruction. Open the images of the GFP channel. Start at time point 1. In the preferred analysis software, use a selection or shape tool to mark each oocyte and generate a region of interest (ROI) for each cell.
- Use the same ROI to select a background area to be subtracted later. Measure GFP pixel intensity in each ROI.
- Using the ROIs for each oocyte generated in step 3.8, measure the GFP intensity in all the time points.
NOTE: In some software programs, an "Analyze" tab allows the selection of the Measure function.- Then, advance to the next time frame and repeat this process to measure the intensity of GFP in each time frame. Lastly, to each GFP value, subtract the measurement of the ROI in the background selected in step 3.8. This analysis will give a value for Securin-gfp intensity for each oocyte at each time point.
- Extract different parameters from the pattern of Securin destruction: (a) the time that Securin-gfp degradation began. This tells when SAC silencing started; (b) the time of Securin-gfp minimum signal. This tells when SAC silencing has dropped below a threshold level to allow high APC/C activation and full Securin-GFP degradation; (c) the rate of Securin-gfp destruction19. This tells how rapidly SAC activity drops below a threshold level for APC/C activation and Securin-GFP degradation.
4. Recruitment of MAD2 at kinetochores by immunofluorescence during meiotic maturation
NOTE: For oocyte collection and maturation, refer to the previously published report12.
- Split oocytes into three groups. Transfer each group of oocytes into 100 µL drops of milrinone-free culture media. Cover the drops with mineral oil and place them in the incubator (37 °C, 5% CO2, and 80% humidity) for 3 h, 5 h, and 7 h to reach early prometaphase I, late prometaphase I, and metaphase I stage, respectively.
NOTE: Place each time point in a different dish to avoid taking out the same dish from the incubator several times, which affects the regular timing of meiotic maturation. - At the assigned time points, fix the oocytes by transferring them into 500 µL drops of 2% PFA in 1x PHEM buffer for 20 min at room temperature, using a 9-well glass dish as previously described20. Then, transfer the cells to a clean well containing a 500 µL drop of blocking solution (PBS + 0.3% BSA + 0.01% Tween-20 + 0.02% NaN3).
NOTE: PHEM composition: 60 mM PIPES; 25 mM HEPES; 10 mM EGTA; and 2 mM MgCl2.
NOTE: One can stop at this point and store the oocytes in blocking solution in the 9-well plate at 4 °C until the convenient time to complete immunofluorescence. - To continue MAD2 detection, transfer the oocytes to a clean well containing a 500 µL drop of permeabilization solution (PBS + 0.3% BSA + 0.1% TritonX-100 + 0.02% NaN3). Incubate for 20 min at room temperature, and then transfer the cells to a new well of blocking solution and incubate for 10 min.
- For the rest of the immunofluorescence steps, use a 96-well dish lid with indentations as previously described20. Use a humidified dark chamber to avoid light exposure and evaporation. Transfer the oocytes into a 30 µL drop of blocking solution with anti-MAD2 antibody (1:1000, rabbit) and anti-centromeric antibody (ACA) (1:30, human) (see Table of Materials) and incubate for 2 h at room temperature.
NOTE: The 96-well dish lid allows room for the processing of several proteins and groups at the same time. - To wash excess primary antibodies, transfer the cells to a 30 µL drop of 1x PHEM buffer supplemented with 0.5% Triton and incubate for 10 min in the humidified chamber. Repeat this step two more times.
- Perform the fourth wash, transferring the cells to a 30 µL drop of 1x PHEM buffer without 0.5% 1x Triton, and incubate for 10 min.
- Transfer the cells to a 30 µL drop of blocking solution containing secondary antibodies such as anti-human-633 (1:200) and anti-rabbit-568 (1:200) (see Table of Materials) and incubate for 1 h at room temperature.
NOTE: Choose the combination of fluorophores based on your microscope lasers or filters. - To wash excess secondary antibodies, repeat steps 4.5-4.6.
- To mount the cells on the microscope slide, transfer the cells to a 10 µL drop of mounting media containing DAPI (0.1 mg/mL) (see Table of Materials). Add small dots of petroleum jelly to each corner of the coverslip, carefully place them on top of the mounting media drop, and press slowly to distribute. Use clear nail polish to seal the coverslip to the slide. For a more detailed description of the mounting process, see reference20.
- Image kinetochores using a confocal microscope (see Table of Materials) equipped with a 40x or 63x objective. Using the ACA signal, determine the z-range that allows imaging of the entire chromosome area.
NOTE: An optical zoom of 4.0 and z-step size of 0.5 µm can be used on some imaging systems. These parameters may vary from system to system, and optimization will be necessary. - Analyze MAD2 intensity at kinetochores using image analysis software. Make a z-stack maximum projection and then split the channels.
- First, create a mask using the ACA channel by selecting the ACA channel to establish a threshold that identifies all the kinetochore signals. Go to the edit tab and create a selection. Then, create an ROI with this selection.
- Select the MAD2 channel and bring in the selection created in step 4.12. Select a threshold method that better adapts to the MAD2 signal. Measure the intensity.
NOTE: Select the threshold method in the control treatment and keep it constant when analyzing different treatments. - To make relative pixel intensity calculations, divide the intensity of each cell by the average intensity of the WT oocytes in the experiment.
Representative Results
Evaluation of SAC responsiveness by nocodazole treatment
The purpose of this experiment is to evaluate SAC activation and strength. By using nocodazole to depolymerize spindle microtubules, all kinetochores will be unattached, which will cause a SAC-mediated cell-cycle arrest. In the present imaging system, DMSO-treated control oocytes extruded a polar body around 14 h after release from milrinone (Figure 1, top panels). Consistent with SAC activation, nocodazole-treated oocytes arrested in metaphase I and did not extrude a polar body (Figure 1, middle panels). To demonstrate that this assay is a reliable indicator of SAC activity, reversine, an MPS1 inhibitor, was used to prevent SAC activation21. When the SAC cannot activate, oocytes extruded a polar body despite having no KT-MT attachments (Figure 1, bottom panels). Oocytes with a weakened SAC will have a mix of outcomes-some oocytes arrest and some extrude polar bodies17. This method of maturing mouse oocytes with and without nocodazole can be used as a first and easy step to challenge SAC activation.
Pattern of Securin-gfp degradation during meiotic maturation
One of the downstream events following SAC satisfaction and silencing is the activation of the APC/C, leading to Securin degradation22. Therefore, evaluation of the pattern of Securin degradation is a direct read-out of SAC function and should be conducted to determine if the nocodazole result was a SAC-dependent perturbation. This assay involves ectopic expression of Securin-gfp into oocytes and subsequent live-cell imaging. After making a curve that follows Securin degradation, three parameters can be determined: (a) The start time of degradation (silencing); (b) the time it takes to reach the minimum Securin level; and (c) the rate of degradation (Figure 2A). In the control, DMSO-treated oocytes, Securin-gfp intensity starts to decrease at ~10 h after milrinone wash out (Figure 2B,C). When oocytes were matured in the presence of the SAC-activating agent nocodazole, Securin-gfp levels were stable during the entire period of meiotic maturation. In contrast, when preventing SAC activation with reversine treatment, the Securin-gfp pattern accelerated; degradation began at ~4 h after milrinone wash-out, consistent with SAC inhibition (Figure 2B,C).
Recruitment of MAD2 to kinetochores by immunofluorescence during meiotic maturation
If the data support a defect in the SAC function, the next step is to evaluate the recruitment of key SAC mediators. The SAC is activated in response to unattached kinetochores. Kinetochores are often unattached in early prometaphase as the spindle is building, and improper attachments are destabilized for correction. Once kinetochores are stably attached to microtubules, the SAC is silenced to allow anaphase onset22. An early step in the SAC response is the recruitment of a series of proteins to kinetochores which serve as a platform for MCC formation22,23. Evaluation of the localization of these SAC components to kinetochores is another strategy to evaluate SAC response. For example, evaluation of MAD2, an MCC component, is a common approach. Confocal microscope images detecting centromeres (ACA) and MAD2 of oocytes matured to early prometaphase I, late prometaphase I, and metaphase I can be used (Figure 3A). To evaluate the strength of the SAC signal, quantify the KT-localized MAD2 pixel intensity (Figure 3B). Significant levels of MAD2 recruitment to KTs occur in early prometaphase I when most KTs are not attached to MTs. The levels of MAD2 reduced in later prometaphase and were nearly absent at metaphase I when all KTs were stably attached to MTs (Figure 3A,B). Therefore, this method can evaluate SAC response when combined with the other two assays.
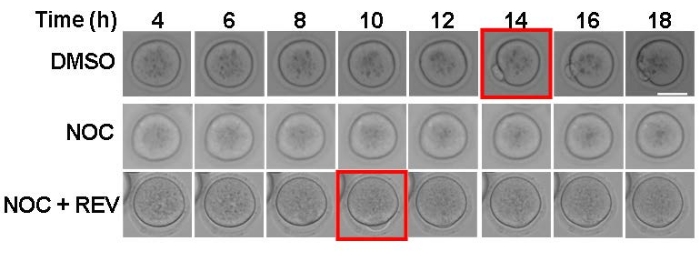
Figure 1: Assessing SAC activation with nocodazole. Representative images from time-lapse imaging of oocytes matured in the presence of DMSO (control), 5 µM nocodazole (NOC), or 5 µM nocodazole + 0.5 µM reversine (NOC + REV). The red square indicates the time frame of polar body extrusion. Two independent experiments were analyzed. Scale bar = 50 µm. Please click here to view a larger version of this figure.
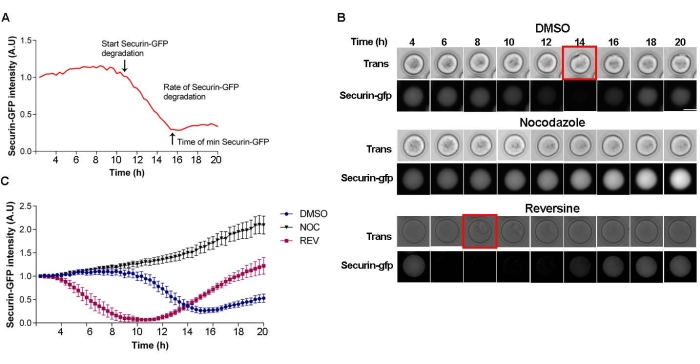
Figure 2: Securin-gfp degradation pattern. (A) Schematic of Securin-gfp degradation curve with three parameters that can be examined. (B) Representative images from a time-lapse of oocytes microinjected with Securin-gfp cRNA and matured in the presence of DMSO (control), 5 µM nocodazole, or 0.5 µM reversine. The red square indicates the time frame of polar body extrusion. Scale bar = 50 µm. (C) Representative Securin-gfp degradation curve of oocytes microinjected with Securin-gfp cRNA and matured in the presence of DMSO (blue circles), 5 µM nocodazole (pink squares), or 0.5 µM reversine (black triangles). Error bars = standard error. # of oocytes per treatment: DMSO = 10; NOC = 15; REV = 5. Please click here to view a larger version of this figure.
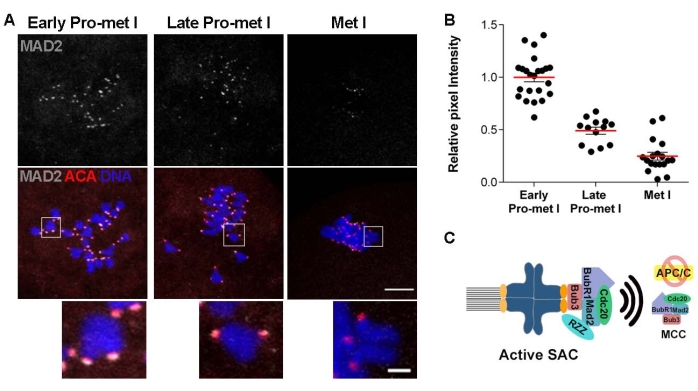
Figure 3: MAD2 recruitment to kinetochores during oocyte meiotic maturation. (A) Representative confocal images of oocytes matured to early prometaphase I, late prometaphase I, and metaphase I, immunostained to detect MAD2 (gray) and kinetochores (ACA) (red). Scale bar = 10 µm; inset: 2 µm. (B) Quantification of the relative intensity of MAD2 from images in (A). Error bars = standard error. (C) Schematic of an unattached kinetochore and SAC activation pathway. # of oocytes per time point: Early prometaphase I = 23; Late prometaphase I = 13; Metaphase I = 18. Please click here to view a larger version of this figure.
Discussion
The spindle assembly checkpoint is a critical control mechanism during cell division designed to prevent chromosome segregation errors. It allows the cell to have enough time to correct improper KT-MT attachments. Meiosis in oocytes is an error-prone process, where most of the chromosome mis-segregation occurs during meiosis I, leading to the generation of aneuploid eggs that are the main cause of early miscarriages and infertility in humans1,24. Several hypotheses are being tested to disentangle why female meiosis has errors. One potential reason is that the SAC is less efficient than in somatic cells and allows anaphase onset with one or more mis-attached KTs9,10,25. Therefore, a comprehensive study of SAC mechanisms in oocytes and identifying key regulators is needed to understand the process of generating a euploid egg.
The present protocol describes three techniques to evaluate different aspects of SAC integrity: activation and silencing. Because each of these assays has some limitations in interpretation, conducting only one is insufficient to characterize SAC function in oocytes. For this reason, performing a combination of these methods to evaluate the SAC integrity in a study is suggested.
Evaluation of SAC activation using nocodazole is straightforward because it is simple and fast. However, there are some important points to consider when interpreting the results. First, the investigator must establish the concentration of nocodazole to completely depolymerize the microtubules of the spindle to generate unattached kinetochores and activate the SAC. Successful loss of microtubules can be determined by fixing oocytes and detecting tubulin via immunofluorescence-based microscopy20. Another critical limitation of this technique is the read-out of this assay: the extrusion of the first polar body (PBE). PBE failure may not necessarily be sufficient evidence of SAC function because if the protein of study affects later stages such as cytokinesis and abscission, the results will also be the failure of PBE. Therefore, use nocodazole treatment as a first step to evaluate SAC defects, but then partner it with the other techniques described.
Once KTs are stably attached to MTs and chromosomes are aligned at the metaphase plate, the SAC is silenced, and the APC/C is activated, leading to Securin and Cyclin B degradation. Therefore, tracking the pattern of Securin or Cyclin B degradation are commonly used indicators of SAC silencing25,26,27. Alteration of the degradation pattern gives information about the cell cycle's acceleration or delay. An acceleration suggests a weak SAC, whereas a delay suggests defects in satisfying the SAC. A limitation of this experiment is that it requires a microinjection rig and a specialized skill set. Note that titration of the proper concentration of Securin-gfp cRNA used for microinjection is critical, as too much will arrest the cell cycle28.
Finally, evaluation of the recruitment of MAD2 to kinetochores is an indicator and measure of SAC activation. The pattern of MAD2 recruitment during meiosis I, with maximum levels of MAD2 at kinetochores at early prometaphase I and reduced levels as KTs become attached. Like the Securin degradation pattern, any alteration of the recruitment of MAD2 to kinetochores could indicate acceleration or delay of SAC silencing. Defects in MAD2 recruitment at early prometaphase could indicate failures in the mechanism of activation or recruitment29 or reduced amounts of MAD2 in the cell17. To distinguish between these two possibilities, evaluating the levels of MAD2 protein expression by Western blot is strongly recommended 17. When comparing two or more treatments, it is important to consider that the timing of meiotic maturation and chromosome alignment could vary among treatments. Therefore, first, define the cell-cycle timing for each treatment to properly compare MAD2 levels and accurately interpret the results. One limitation of this method is that MAD2 is one of the last effectors of the SAC response3. Therefore, assessing MAD2 does not allow one to pinpoint which part of the SAC mechanism is perturbed. To evaluate SAC mechanisms, one can determine the recruitment of another SAC component such as MPS1, BUB1, and/or the RZZ complex (Figure 3C). Another point to consider is that the fixation step is critical. These assays used 2% PFA in 1x PHEM buffer, a fixation that works for the detection of kinetochore proteins in whole mount oocytes. However, other laboratories use a chromosome spread technique that was extensively described before30.
In summary, three assays, that provide critical information about a cell's ability to monitor kinetochore-spindle microtubule attachment status to control chromosome segregation accurately are described in this article. This information is important for investigating cellular situations where the SAC may be compromised to gain insight into which step of the checkpoint is perturbed. Because aneuploidy is a common feature in gametes and cancers, these tools apply to many biological systems.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
Funding for this project was provided by the National Institutes of Health (R35GM136340 to KS).
Materials
| Bovine serum albumin (BSA) | Sigma | A4503 | |
| DAPI | Life Technologies | D1306 | |
| Dimethyl-sulfoxide (DMSO) | Sigma | D5879 | |
| Donkey-anti-rabbit-Alexa-568 | Life Technologies | A10042 | |
| EVOS FL Auto Imaging System | Life Technologies | Fluorescence microscope | |
| EVOS Onstage Incubator | Life Technologies | Incubator chamber | |
| Glass Bottom 96- well plates N 1.5 uncoated | MatTek Corporation | P96G-1.5-5-F | |
| goat-anti-human-Alexa-633 | Life Technologies | A21091 | |
| HEPES | Sigma | H3537 | |
| Human anti-ACA | Antibodies Incorporated | 15-234 | Dilution 1/30 |
| ImageJ | NIH | ||
| KCl | Sigma | P5405 | |
| KH2PO4 | Sigma | P5655 | |
| Leica SP8 equipped with a 63×, 1.40 NA oil immersion objective | Leica | ||
| MgSO4·7H20 | Sigma | M7774 | |
| Milrinone | Sigma | M4659 | |
| Na2HPO4 | Sigma | S2429 | |
| NaCl | Sigma | S5886 | |
| NaN3 | Sigma | S2002 | |
| Nocodazole | Sigma | M1404 | |
| Paraformaldhyde (PFA) | Sigma | P6148 | |
| PIPES | Sigma | P6757 | |
| Rabbit anti- MAD2 | Biolegend | 924601 | Dilution 1/1000; previously Covance #PRB-452C |
| Reversine | Cayman Chemical | 10004412 | |
| Triton-X | Sigma | 274348 | |
| Tween-20 | Sigma | X100 | |
| Vectashield | Vector laboratories | H-1000 |
Referencias
- Gruhn, J. R., et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 365 (6460), 1466-1469 (2019).
- Nagaoka, S. I., Hassold, T. J., Hunt, P. A. Human aneuploidy: mechanisms and new insights into an age-old problem. Nature Reviews. Genetics. 13 (7), 493-504 (2012).
- Musacchio, A. The molecular biology of spindle assembly checkpoint signaling dynamics. Current Biology. 25 (20), 1002-1018 (2015).
- Lara-Gonzalez, P., Westhorpe, F. G., Taylor, S. S. The spindle assembly checkpoint. Current Biology. 22 (22), 966-980 (2012).
- London, N., Biggins, S. Signalling dynamics in the spindle checkpoint response. Nature Reviews Molecular Cell Biology. 15 (11), 736-748 (2014).
- Kuhn, J., Dumont, S. Mammalian kinetochores count attached microtubules in a sensitive and switch-like manner. Journal of Cell Biology. 218 (11), 3583-3596 (2019).
- Jones, K. T., Lane, S. I. R. Molecular causes of aneuploidy in mammalian eggs. Development. 140 (18), 3719-3730 (2013).
- Gui, L., Homer, H. Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes. Development. 139 (11), 1941-1946 (2012).
- Nagaoka, S. I., Hodges, C. A., Albertini, D. F., Hunt, P. A. Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Current Biology. 21 (8), 651-657 (2011).
- Sebestova, J., Danylevska, A., Novakova, L., Kubelka, M., Anger, M. Lack of response to unaligned chromosomes in mammalian female gametes. Cell Cycle. 11 (16), 3011-3018 (2012).
- Vasquez, R. J., Howell, B., Yvon, A. M., Wadsworth, P., Cassimeris, L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Molecular Biology of the Cell. 8 (6), 973-985 (1997).
- Stein, P., Schindler, K. Mouse oocyte microinjection, maturation and ploidy assessment. Journal of Visualized Experiments. (53), e2851 (2011).
- Thomas, R. E., Thompson, J. G., Armstrong, D. T., Gilchrist, R. B. Effect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacity. Biology of Reproduction. 71 (4), 1142-1149 (2004).
- Marin, D., Nguyen, A. L., Scott, R. T., Schindler, K. Using mouse oocytes to assess human gene function during meiosis I. Journal of Visualized Experiments. (134), e57442 (2018).
- Herbert, M., et al. Homologue disjunction in mouse oocytes requires proteolysis of securin and cyclin B1. Nature Cell Biology. 5 (11), 1023-1025 (2003).
- Solc, P., et al. Multiple requirements of PLK1 during Mouse oocyte maturation. PLoS One. 10 (2), 0116783 (2015).
- Blengini, C. S., Nguyen, A. L., Aboelenain, M., Schindler, K. Age-dependent integrity of the meiotic spindle assembly checkpoint in females requires Aurora kinase B. Aging Cell. 20 (11), 13489 (2021).
- Balboula, A. Z., et al. Haspin kinase regulates microtubule-organizing center clustering and stability through Aurora kinase C in mouse oocytes. Journal of Cell Science. 129 (19), 3648-3660 (2016).
- Nabti, I., Grimes, R., Sarna, H., Marangos, P., Carroll, J. Maternal age-dependent APC/C-mediated decrease in securin causes premature sister chromatid separation in meiosis II. Nature Communications. 8 (1), 15346 (2017).
- Blengini, C. S., Schindler, K. Immunofluorescence technique to detect subcellular structures critical to oocyte maturation. Methods in Molecular Biology. 1818, 67-76 (2018).
- Santaguida, S., Tighe, A., D’Alise, A. M., Taylor, S. S., Musacchio, A. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. Journal of Cell Biology. 190 (1), 73-87 (2010).
- Musacchio, A. The molecular biology of spindle assembly checkpoint signaling dynamics. Current Biology. 25 (20), 1002-1018 (2015).
- Wassmann, K., Niault, T., Maro, B. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Current Biology. 13 (18), 1596-1608 (2003).
- Hassold, T., Hall, H., Hunt, P. The origin of human aneuploidy: where we have been, where we are going. Human Molecular Genetics. 16, 203-208 (2007).
- Gui, L., Homer, H. Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes. Development. 139 (11), 1941-1946 (2012).
- Homer, H. A., et al. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes and Development. 19 (2), 202-207 (2005).
- Blengini, C. S., et al. Aurora kinase A is essential for meiosis in mouse oocytes. PLOS Genetics. 17 (4), 1009327 (2021).
- Hagting, A., et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. Journal of Cell Biology. 157 (7), 1125-1137 (2002).
- Rodriguez-Rodriguez, J. A., et al. Distinct roles of RZZ and Bub1-KNL1 in mitotic checkpoint signaling and kinetochore expansion. Current Biology. 28 (21), 3422-3429 (2018).
- Chambon, J. P., Hached, K., Wassmann, K. Chromosome spreads with centromere staining in mouse oocytes. Methods in Molecular Biology. 957, 203-212 (2013).

