Study of the Functions and Activities of Neuronal K-Cl Co-Transporter KCC2 Using Western Blotting
Summary
The present protocol highlights the application of western blotting technique to study the functions and activities of neuronal K-Cl co-transporter KCC2. The protocol describes the investigation of KCC2 phosphorylation at kinase regulatory sites Thr906/1007 via western blotting. Also, additional methods to confirm KCC2 activity are briefly highlighted in this text.
Abstract
Potassium chloride cotransporters 2 (KCC2) is a member of the solute carrier family 12 (SLC12) of cation-chloride-cotransporters (CCCs), found exclusively in the neuron and is essential for the proper functioning of Cl– homeostasis and consequently functional GABAergic inhibition. Failure in proper regulation of KCC2 is deleterious and has been associated with the prevalence of several neurological diseases, including epilepsy. There has been considerable progress with regard to understanding the mechanisms involved in the regulation of KCC2, accredited to the development of techniques that enable researchers to study its functions and activities; either via direct (assessing kinase regulatory sites phosphorylation) or indirect (observing and monitoring GABA activity) investigations. Here, the protocol highlights how to investigate KCC2 phosphorylation at kinase regulatory sites – Thr906 and Thr1007- using western blotting technique. There are other classic methods used to directly measure KCC2 activity, such as rubidium ion and thallium ion uptake assay. Further techniques such as patch-clamp-electrophysiology are used to measure GABA activity; hence, indirectly reflecting activated and/or inactivated KCC2 as informed by the assessment of intracellular chloride ion homeostasis. A few of these additional techniques will be briefly discussed in this manuscript.
Introduction
Potassium chloride cotransporters 2 (KCC2) is a member of the solute carrier family 12 (SLC12) of cation-chloride-cotransporters (CCCs), found exclusively in the neuron and is essential for the proper functioning of Cl– homeostasis and consequently functional GABAergic inhibition1,2,3,4. The maintenance of low intraneuronal Cl– concentration ([Cl–]i) at 4-6 mM by KCC2 facilitates γ-aminobutyric acid (GABA)/glycine hyperpolarization and synaptic inhibition in the brain and spinal cord5. Failure in the proper regulation of KCC2 has been associated with the prevalence of several neurological diseases, including epilepsy4. Furthermore, decreased KCC2-mediated Cl− extrusion and impaired hyperpolarizing GABAA and/or glycine receptor-mediated currents have been implicated in epilepsy, neuropathic pain, and spasticity6,7. Neuronal KCC2 is negatively modulated via phosphorylation of key regulatory residues within its C-terminal intracellular domain by the with-no-lysine (WNK)-STE20/SPS1-related proline/alanine-rich (SPAK)/Oxidative stress-responsive (OSR) kinase signaling complex1, which facilitates the maintenance of depolarized GABA activity in immature neurons2,8,9. The WNK-SPAK/OSR1 phosphorylates threonine residues 906 and 1007 (Thr906/Thr1007) and subsequently downregulates mRNA gene expression of KCC2, leading to a consequent deterioration in its physiological function8,10. More importantly, however, it is already a fact that the WNK-SPAK/OSR1 kinase complex is known to phosphorylate and inhibit KCC2 expression1,2,4,11,12, and that the inhibition of the kinase complex signaling pathways to phosphorylate Thr906/Thr1007 has been linked with the increased expression of the KCC2 mRNA gene13,14,15. It is important to note that the regulation of neuronal KCC2 and Na+-K+-2Cl– cotransporters 1 (NKCC1) expression via protein phosphorylation works concomitantly and in converse patterns1,4,16.
There has been consistent and considerable progress with regard to the understanding of mechanisms involved in the regulation of KCC2, accredited to the development of techniques that enables researchers to study its functions and activities; either via direct (assessing kinase regulatory sites phosphorylation) or indirect (observing and monitoring GABA activity) investigations. The protocol presented here highlights the application of western blotting techniques to study the functions and activities of neuronal K+-Cl– co-transporter KCC2 by investigating the phosphorylation of the cotransporter at kinase regulatory sites Thr906/1007.
Western blot is a method used to detect specific proteins of interest from a sample of tissue or cell. This method first separates the proteins by size through electrophoresis. The proteins are then electrophoretically transferred to a solid support (usually a membrane) before the target protein is marked using a specific antibody. The antibodies are conjugated to different tags or fluorophore-conjugated antibodies that are detected using either colorimetric, chemiluminescence, or fluorescence methods. This allows for a specific target protein to be detected from a mixture of proteins. This technique has been used to characterize phosphospecific sites of KCCs1 and has been used to identify kinase inhibitors that inhibit KCC3 Thr991/Thr1048 phosphorylation17.By following this protocol, one can specifically detect total and phosphorylated KCC2 from cell/tissue lysates. In principle, the detection of protein-conjugated antibodies by this technique is highly instrumental as it helps to improve the understanding of cooperative activities at the phospho-sites of KCC2, which sheds light on the molecular mechanisms involved in their physiological regulations. The quantitative analysis of the total protein expression is representative of the function and activity of KCC2. There are other classical methods used to directly measure KCC2 activity, such as rubidium ion and thallium ion uptake assay. Further techniques such as patch-clamp-electrophysiology are used to measure GABA activity; hence, indirectly reflecting activated and/or inactivated KCC2 as informed by the assessment of intracellular chloride ion homeostasis.
Protocol
NOTE: The protocol describes the western blotting method to detect specific proteins of interest.
1. Cell culture and transfection
- Warm all the reagents in the bead bath (37 ˚C) before the cell culture procedure. Prepare culture medium, Dulbecco's Modified Eagle Medium (DMEM), supplemented with 10% fetal bovine serum, 1% each of 2mM L-glutamine, 100x non-essential amino acid, 100 mM sodium pyruvate, and 100 units/mL penicillin-streptomycin.
- Thaw stably transfected rat KCC2b human embryonic kidney 293 cells (HEK293rnKCC2b)18 completely in the bead bath (37 ˚C). Transfer the cells from the cryovial tube into a centrifuge tube containing 5 mL of fresh media. Spin the cell at 1200 ×g for 3-5 min.
- Use an aspirator pipette (fixed to a vacuum pump) to aspirate the supernatant and add 10 mL of fresh media to re-suspend the cells. Transfer the cell suspension to a 10 cm dish plate.
- Place the dish into the incubator and allow it to grow for 48 h at 37 °С in a humidified 5% CO2 atmosphere. Monitor the incubation period to ensure healthy cell growth. Further split the cells when they have attained over 90% confluence after the incubation period.
- Aspirate the old media out from the cells dish and gently rinse cells with 2 mL of phosphate buffer saline (PBS). Add 2 mL of trypsin and incubate for about 1-2 min at room temperature.
- Use 2 mL of complete media to gently wash the trypsinized cells in the dish. Add 9 mL of fresh media to new dishes and add 1 mL solution from the old dish to each of the new ones. Transfer the split cell dishes back to the incubator for 48 h to attain ≥ 90% confluency.
- Treat the cells with either dimethyl sulfoxide (DMSO) as control, 8 µM staurosporine, or 0.5 mM N-ethylmaleimide (NEM) for 15 min before harvesting the cells in lysis buffer.
2. Preparation of cell lysates and loading samples
- Aspirate the media on the culture dish (from transfection procedures). Place the cell culture on ice and wash the cells with ice-cold PBS.
- Aspirate the PBS, then add 1.0 mL of ice-cold lysis buffer containing 50 mM tris-HCl (pH 7.5), 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM ethylenediaminetetraacetic acid (EDTA), 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 10 mM sodium-β-glycerophosphate, 1 mM sodium orthovanadate, 1% (w/v) Triton X-100, 0.27 M sucrose, 1 mM benzamine, 0.1% (v/v) 2-mercaptoethanol, and 2 mM phenylmethylsulfonylfluoride (PMSF) to the dish.
NOTE: The volume of lysis buffer during cell harvest varies with dish sizes, for example, 1 mL of lysis buffer is suitable per 1 x 107 cells/100 mm dish/150 cm2 flask, while 0.5 mL is suitable per 5 x 106 cells/60 mm dish/75 cm2 flask. - Use a cold plastic cell scraper to scrape the cells off the bottom of the dish, then gently use a pipette to transfer the cell suspension into a micro-centrifuge tube already on ice. Constantly agitate the tube for 30 min at 4 °C.
- Spin the cell lysates in a cold centrifuge (4 °C) at 16,000 x g for 20 min, remove the tube gently from the centrifuge, and place it on ice. Collect the supernatant into a pre-cooled fresh tube and discard the pellet.
NOTE: It may be necessary to vary the centrifugation force and time depending on the cell type. Though, general guidelines encourage centrifugation at 16,000 x g for 20 min. However, this must be determined for every experiment. For instance, delicate cells like leukocytes need a very light centrifugation speed.
3. Preparation of immunoprecipitants from cell lysates
- Pipette 300 µL of protein-G-sepharose into a microcentrifuge tube. Spin the solution at 500 x g for 2 min and discard the supernatant.
- Add 500 µL of PBS and vortex the solution well. Spin the solution at 500 x g for 2 min and discard the supernatant. Repeat this step.
- Mix 1 mg of anti-KCC2 Thr906 and anti-KCC2 Thr1007 antibodies with 200 µL of protein G-sepharose beads and make up the volume to 500 µL with PBS. Shake on a vibrating platform or rotating wheel for 2 h at 4 °C. Wash 2x with PBS.
- Carry out protein quantification on the whole cell lysates and add 1 mg of the cell lysate to the washed beads. Gently incubate in an end-to-end rotator for 2 h at 4 °C. Spin down the beads and wash them 3x with PBS containing 150 mM sodium chloride (NaCl).
- Wash immunoprecipitants (beads) 3x with 200 µL of PBS. Resuspend the final pellet in 100 μL of 1x lithium dodecyl sulfate (LDS) sample buffer.
- Shake the tubes in a rotor shaker at room temperature for 5 min and incubate them in a heating block at 75 °C for 10 min. Centrifuge the loading sample at 11,000 x g for 2 min and use the supernatant for gel loading.
4. Performing the western blot
- Assemble the casting apparatus and pour freshly prepared 8% separating gel (see Table 1 for recipe/preparation) to cast the gel allowing about 2 cm of space from the top of the casting glass. Add 200 µL of absolute isopropanol to the setup and allow it to stand at room temperature for 60 min.
NOTE: The polyacrylamide gel recipe for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) depends on the size of the protein of interest (Table 2). Hence, make a note of the protein size before determining the desired gel percentage. - Use a pipette to remove the isopropanol and carefully rinse the gel with about 200 µL of distilled water. Add freshly prepared 6% stacking gel (see Table 1 for recipe/preparation) to fill up the approximately 2 cm space on the casting setup. Gently fit in the well comb and allow to stand at room temperature for 30 min.
- Fix the casted gel into the electrophoresis tank.
- Dissolve 30.3 g of Tris base, 144.1 g of glycine, and 10 g of SDS in 1000 mL of distilled water to prepare 10x transfer buffer. Prepare 1x running buffer by adding 10 mL of 10% SDS and 100 mL of 10x transfer buffer to 890 mL of distilled water.
- Pour 1x running buffer into the tank. Load 5 µL of molecular weight marker into the first well and an equal amount of protein into each well of the SDS-PAGE gel (between 18-30 µL, depending on the comb size used). Fill up empty wells with 1x LDS and run the gel for about 90-120 min at 120 V.
- Dissolve 58.2 g of Tris base and 29.3 g of glycine in 1000 mL of distilled water to prepare 10x transfer buffer. Activate nitrocellulose membrane with 1x transfer buffer containing 20% methanol. Rinse the gel and membrane with the transfer buffer and gently spread them out on the preparing stack.
NOTE: There is no need to adjust the pH of the running and transfer buffers as they should be at the optimum pH required. Also, it is advisable to prepare these buffers and store them at room temperature before the experiment to save time. - Arrange the sandwich to be transferred in this order: negative electrode (black frame/end) – sandwich foam – filter paper – rinsed SDS-PAGE gel – rinsed nitrocellulose membrane – filter paper – sandwich foam – positive electrode (red frame). Stack the assembled sandwich in the transfer tank and run at 90 V for 90 min or 30 V for 360 min.
5. Antibody staining and image development
- Remove the membrane and let it dry. Block the membrane for 1 h at room temperature using a blocking buffer made from 5% skimmed milk in Tris-buffered saline containing 0.1% 1x Tween 20 (1x TBS-T).
- Incubate the membranes with appropriate dilutions of primary antibody8,15,19 and beta-actin (loading control) in blocking buffer for 1 h at room temperature or overnight at 4 °C. Wash the membrane in three washes of 1x TBS-T for 5 min each.
NOTE: The incubation of the separate membrane with loading controls such as beta-actin, alpha-tubulin, or glyceraldehyde 3-phosphate dehydrogenase antibodies is to normalize the other western blotting results during subsequent quantification. The recommended dilution for each primary antibody is available in the manufacturer's manual. - Incubate the washed membrane with secondary antibody diluted 5000-fold in blocking buffer for 60 min at room temperature. Again, wash the membrane 3x in 1x TBS-T for 5 min each.
NOTE: It is highly recommended that the secondary antibody must be raised against the species in which the primary antibody is raised. For example, if the primary antibody of total KCC2 is mouse anti-KCC2, then the secondary antibody for anti-mouse must be used. - Place the washed membrane on the imaging board. Mix equal volumes of each enhanced chemiluminescence (ECL) reagent and gently spread the mixed solution on the membrane to develop signals.
6. Image acquisition using an imaging system and data quantification
- Transfer the imaging board to the appropriate compartment on the imaging system for imaging. Open the imaging software on the computer for image processing.
- On the toolbar, click New Protocol and choose Single Channel. Click Select under gel imaging/application dialog box, scroll to Blot and select either Chemi Hi Sensitivity or Chemi Hi Resolution. Then click on Signal Accumulation Setup to set the duration and number of images to acquire.
- Click Position Gel under protocol setup and adjust the gel in the imaging system if necessary. Click Run Protocol to acquire the images.
- Right-click on one of the acquired images under the imaging catalog box to save the images on the computer. Navigate to the desired image and click on Select Image and Continue under the run dialog box.
- Click on the Image Transform icon to adjust the contrast and the pixel saturation of the image. Click on the Screenshot icon on the general toolbar to save the image to the computer depending on the desired image format.
- Measure the band densities using ImageJ software and analyze using appropriate statistical tools.
Representative Results
Here, the representative result presented in Figure 1 investigated the impact of staurosporine and NEM on WNK-SPAK/OSR1 mediated phosphorylation of KCC2 and NKCC1 in HEK293 cell lines stably expressing KCC2b (HEKrnKCC2b)18 using the western blotting technique. Comprehensive details on the representative results are discussed in Zhang et al.15. Similar to NEM, staurosporine is a broad kinase inhibitor that can enhance KCC2 transport activity and inhibit NKCC1 activity15,20. The treatment of HEK293 cells with staurosporine and NEM caused decreased phosphorylation at SPAK target sites – Thr233 situated in the T-loop kinase domain and the S-loop phosphorylation site Ser373 of hsSPAK. Thus, both compounds abridged the phosphorylation levels of the aforementioned SPAK phospho-sites. Also, staurosporine reduced the phosphorylation of Ser940 in HEKrnKCC2b, but NEM significantly increased its phosphorylation. Furthermore, staurosporine decreases phosphorylation at the Thr505 site, whereas NEM caused a slight, but insignificant increase in phosphorylation at the same site. The differential effects of both compounds on the phosphorylation of protein kinase C (PKC) at the Thr505 site, and KCC2b at the Ser940 site correlate well. The representative result also showed that both agents altered the expression of total rnKCC2b, hsNKCC1, or hsSPAK. NEM (but not staurosporine treatment) caused a significant increase in the expression of the total KCC2 amount, whereas the expression of total NKCC1 and SPAK were not significantly changed when treated with both compounds. Furthermore, the two compounds caused a decreased phosphorylation of SPAK at Thr233 and Ser373 sites, and this correlated with the abridged phosphorylation of Thr906/Thr1007 and Thr203/207/212 sites in rnKCC2b and hsNKCC1, respectively. Additionally, staurosporine and NEM reduced and increased the phosphorylation of PKC at the Ser940 site in rnKCC2b, respectively, which correlated with the reduction and increment in the phosphorylation of PKC-δ at the Thr505 site upon staurosporine and NEM treatment, respectively (Figure 1).
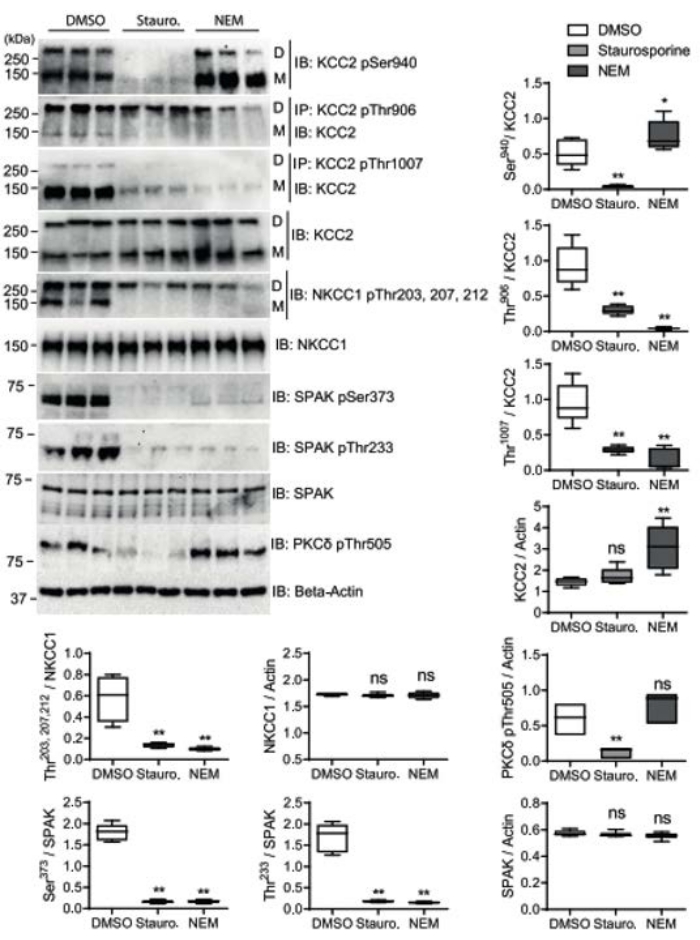
Figure 1: Quantitative analyses of rnKCC2b and hsNKCC1 phospho-sites upon staurosporine and NEM treatment in HEKrnKCC2b cells. (A) Stably transfected HEKrnKCC2b cells were treated with DMSO (control), 8 µM staurosporine, or 0.5 mM NEM for 15 min. Cell lysates were harvested and subjected to immunoprecipitation (IP) and immunoblot (IB) with indicated antibodies. Abbreviations: D = dimeric KCC2; M = monomeric KCC2. (B) Immunoblot quantification of stably transfected HEKrnKCC2b cells. Band intensities were quantified with ImageJ software. ***p < 0.001; **p < 0.01; Wilcoxon-Mann Whitney test (n = 6). This figure has been modified from Zhang et al.15. Please click here to view a larger version of this figure.
| 8 mL of 8% Separating Gel | 5 mL of 6% Stacking Gel | ||
| Materials needed | Volume (µL) | Materials needed | Volume (µL) |
| Distilled H2O | 4200 | Distilled H2O | 2900 |
| 40% Acrylamide | 1600 | 40% Acrylamide | 750 |
| 1.5 M Tris pH 8.8 | 2000 | 0.5 M Tris pH 6.8 | 1250 |
| 10% SDS | 80 | 10% SDS | 50 |
| 10% APS | 80 | 10% APS | 50 |
| TEMED | 8 | TEMED | 5 |
| Making the separating gel: | |
| 1) | Begin with 4.2 mL of ddH2O |
| 2) | Add 1.6 mL of 40% acrylamide/bis-acrylamide solution |
| 3) | Add 2 mL of 1.5 M Tris pH 8.8 and mix |
| 4) | Mix in 80 µL of 10% SDS |
| 5) | When ready to use, add 8 µL of TEMED and mix |
| 6) | Add 80 µL of 10% APS* and mix |
| Making the stacking gel: | |
| 1) | Begin with 2.9 mL of ddH2O |
| 2) | Add 0.75 mL of 40% acrylamide/bis-acrylamide solution |
| 3) | Add 1.25 mL of 0.5 M Tris pH 6.8 and mix |
| 4) | Mix in 50 µL of 10% SDS |
| 5) | When ready to use, add 5 µL of TEMED and mix |
| 6) | Add 50 µL of 10% APS* and mix |
| SDS = Sodium Dodecyl Sulfate | |
| APS = Ammonium Per Sulfate | |
| TEMED = N, N, Nʹ, Nʹ-tetramethylethylenediamine | |
| *Upon addition of APS, the solution should be immediately poured into the casting apparatus because the solution polymerizes within few minutes. Hence, it is advisable to prepare the separating and stacking gel solutions just about when the solutions are needed. | |
Table 1: Recipe for making polyacrylamide gel mix (separating and stacking gel solutions).
| Gel Percentage (%) | Protein Size (kDa) |
| Up to 20 | 4 to 40 |
| 15 | 12 to 45 |
| 12.5 | 10 to 70 |
| 10 | 15 to 100 |
| 8 | 50 to 200 |
| 4 to 6 | > 200 |
Table 2: Recommended SDS-PAGE gel percentage for different sizes of proteins
Discussion
Many methods have been used to measure the activities of SLC12 of CCCs that are expressed in the neurons, including KCC2. Many of these techniques have proven to enhance scientific knowledge on the analysis of the functional relevance of these transporters and their structure-function patterns in different disease-related mutations. Critically, there are advantages and caveats to the various methods21. However, the protocol explained above, outlined how to assess KCC2 phosphorylation at kinase regulatory sites, Thr906 and Thr1007, using western blot, which can be helpful in studying the functions and activities of KCC2.
The regulation of KCC2 occurs through phosphorylation and dephosphorylation at key serine/threonine residues1. Western blotting is an efficient technique that can be used to investigate KCC2 phosphorylation at kinase regulatory sites at Thr906 and Thr1007. In principle, changes in the phosphorylation of KCC2 are detected using phospho-specific antibodies directed against phospho-peptides of the immunoblot samples/proteins of interest. Western blot is a method used to detect the specific protein of interest from a sample of tissue or cell. This method first separates the proteins by size through electrophoresis. The proteins are then electrophoretically transferred to a solid support (usually a membrane) before the target protein is marked using a specific antibody. The antibodies are conjugated to different tags or fluorophore-conjugated antibodies that are detected using either colorimetric, chemiluminescence, or fluorescence methods. This allows for a specific target protein to be detected from a mixture of proteins. This technique has been used to characterize phosphospecific sites of KCC22,8 and has been used to identify inhibitors of KCC2 that antagonize KCC2 Thr906/Thr1007 phosphorylation15. HEK293 lines have been in use for several experiments owing to their advantage in molecular biology experiments. They are quick to reproduce, easy to maintain, and highly efficient for transfection and protein production22. However, some opinions hold that it might not be the most suitable candidate for carrying out neurobiology experiments because it is not a cell sourced from the brain, and hence its physiological specificity may be questionable in neuroscience research. However, previous works have shown that similar results are observed in the expression of KCC2 in HEK293 cells and actual neuronal tissues/cells15,23. Hence, the relevance of HEK293 in neuroscience research may not be discarded outright.
In studies involving the analysis of protein expression by western blotting technique, specific attention should be given to the choice of lysis buffer composition, the nature (type and concentration) of the detergent to be used24, and the protease inhibitors. The composition of the buffer must be optimized to suit the target protein to facilitate its solubilization and extraction processes, and to allow its easy detection by western blot25. Mild detergent at low concentration is usually required for soluble proteins, while membrane proteins may require stronger detergent conditions. However, strong detergents may disrupt interactions and complexes may be lost. Both types and concentrations of the detergents may affect the nature and activity of the protein, hence, there is a need to stick to restricted/tolerated range of concentrations for these detergents. The addition of protease inhibitor will avert the target protein from being degraded by endogenous proteins, and the optimal recommended working temperature is 4 °C to effectively decline proteolytic rates. Sometimes, in a bid to achieve high-quality immunoblot signals for antibodies that give poor immunoblot signals, immunoprecipitation (IP) is carried out as an upstream experiment for western blotting. This technique is advantageous because it facilitates the interaction between antigens and their respective antibodies in their native conformation preceding their consequent separation and quantification26. Hence, IP may enhance the quality of outputs from western blotting procedures. Prior to the application of the IP technique, it is important to assess the presence and efficiency of expression of the co-immunoprecipitated partner proteins in the lysate by analyzing either the whole cell lysate or input fractions by western blotting. Furthermore, prior knowledge of the molecular weight of the protein to be investigated is necessary before preparing the desired percentage gel solutions for SDS-PAGE electrophoresis as the size of the protein impacts the sieving effect of the gel matrix.
Though, the detection of protein-conjugated antibodies by this technique is highly instrumental as it helps to improve the understanding of cooperative activities at the phospho-sites of KCC2 which may provide information about the cotransporter phosphorylation and the integrity of the signaling pathway that may regulate the cotransporters, for a reliable prediction of cotransporter activity. However, it is important to note that the western blot technique is only able to produce semi-quantitative data, which means that the technique only highlights the relative assessment of protein expressions but not absolute quantification. This is accredited to discrepancies in the rates of sample loading and transfer in separate lanes, which are different on individual blots. The detected signal generated is also not linear and does not reflect the concentration range of samples27. Besides, phosphorylation status may not be a reliable factor for reporting protein activity as interventions such as inhibitors, mutations, and protein interactions with its environment can directly influence protein activity without changing its phosphorylation level28,29,30. Thus, it may be more beneficial to use phospho-specific antibodies to supplement other biochemical methods.
As mentioned earlier, there are other classic methods used to directly measure KCC2 activity. The assessment of KCC2 through measuring radioactive 86Rb+ flux through the membrane of homogenous cell preparations is a popular approach. This method uses active radioisotopes as tracers by passing them through ionic channels. Briefly, the cells of interest are incubated in cation free solutions to inhibit endogenous KCC2, followed by incubation with specific inhibitors such as ouabain, and then with uptake medium containing the inhibitor and 86Rb+. Liquid scintillation counters are used to measure/trace activities sequel to cell lysis. Though, this method is robust, highly sensitive and selective and is less prone to disturbances. However, its applications do not extend to tissues with multiple cell types like brain or neuron cultures. Moreover, this technique restricts changes in the resolution of ion concentration at the subcellular level. Furthermore, there are safety issues regarding the potential toxicity and health hazard of working with short half-life (18.65 days) radioisotopes characterized with high energy emission (max 1.77 MeV; max 1.08 MeV)31. This could massively suggest an unsuitability for neuronal cells studies where high number of cells are usually required. These issues lead to the development of the non-radioactive 85Rb+ efflux assay by Terstappen 1999, as an alternative to the radioactive 86Rb+ for determination of ion fluxes. The non-radioactive approach has greatly exiled the radioactive 86Rb+ assays for the analysis of K+ and non-selective cation channels in the pharmaceutical industry31. Non-radioactive 85Rb+ efflux assay involves two major processes, the first is cell culture and manipulation and the second is the determination of the tracer rubidium by atomic absorption spectroscopy (AAS). This method is easy to use, though, it does require a number of assay validation experiments and suffer from poor temporal resolution32. Nevertheless, non-radioactive 85Rb+ efflux assay has progressively proved to be a reliable technique for the assessment of KCCs and NKCCs33.
The thallium (TI+) flux assay uses TI+ as a surrogate ion for K+ due to its high binding affinity to the K+ site on KCC2 and NKCC2. TI+ influx into cells could be detected using a thallium-sensitive fluorescent dye such as benzothiazole coumarin and fluozin-2. Once transported by the channel, TI+ may associate with the BTC/fluozin-2 dye causing a fluorescent change that can be detected by fluorometric imaging plate reader31. TI+ indicator dyes enter cells as acetoxymethyl esters which are then cleaved by cytoplasmic esterases to release the active fluorogenic forms. To activate the KCCs, the cells are stimulated with a mixture of K+ and Tl+ in the presence or absence of test compounds (e.g., KCC2 inhibitors). Thallium enters and binds to the fluorescence dye. The increase in fluorescent signal is proportional to the influx of Tl+ into the cell specifically through the cotransporter, and therefore represents a functional measurement of the cotransporter activity. This method has also been well applied in measuring KCC2 and NKCC2 activity in various types of cell cultures, for examples, studies on HEK293 cell line stably expressing human KCC234. On a demerit end, there is a variety of potential off-target pathways lines that could interfere with TI+ influx, for example, Na+/K+ ATPase in HEK293 cells could cause a higher false positive or false negative hit rate35. Moreover, as with other flux assays, the implementation of such approach in neuronal cells remain limited due to the poor survival of neuronal cells after multiple washing steps. The neuronal expression of multiple K+ channels and transporter can also impede the accurate assessment of KCC2 specific K+ fluxes. This technique present limitations for study of channels in small neuronal compartment such as dendrites and dendritic spines, and the axons owed to lack of spatial resolution coupled with low temporal resolution.
Further techniques such as patch-clamp-electrophysiology are used to measure GABA activity; hence, indirectly reflecting activated and/or inactivated KCC2 as informed by the assessment of intracellular chloride ion homeostasis. Generally, electrophysiological measurements of SLC12 function rely on the principle that the GABAA receptors (GABAAR) are permeable to chloride36. KCC2 is key in the modulation of GABAergic inhibition. Particularly, the [Cl–]i extrusion activity of KCC2 underlies the hyperpolarization of GABAAR6. Intracellular recordings provide crucial insights through the extrapolation of chloride extruding capacity of KCC2 from the reversal in the potential of GABAAR-mediated currents (EGABA). A hyperpolarized EGABA indicates lower [Cl–]i and thus an increase in the activity of KCC2. Gramicidin patch-clamp technique in electrophysiology is a gold standard approach for measuring GABA activity. Some previously published studies have employed gramicidin patch-clamp recording to investigate the functions and activities of KCC2 to assess/monitor [Cl–]i homeostasis have indeed proven successful8,9,37,38,39,40. During a gramicidin patch-clamp recording, a tight seal on the cell/tissue surface is created using a glass electrode with a relative patch to record the intracellular activity of ion channels in reference to another electrode in a bath surrounding the cell/tissue. This technique can be performed by measuring the amount of current that crosses a cell's membrane at a fixed voltage in the voltage-clamp mode or by recording the amount of voltage moving across the membrane at a fixed current in the current clamp mode36. Several variations of the basic technique can be applied which largely depends on the research question. The technique does not create a relatively large pore-like whole-cell patch recording but the pore-forming antibiotic (gramicidin) that is contained in the electrode solution is used to form small pores in the membrane36. Particularly, the addition of gramicidin creates cation-selective pores in the membrane, which exhibit insignificant anion permeability. Voltage ramp protocols are an effective and reliable alternative to recording the current at a steady voltage. The current/voltage relations obtained with voltage ramp protocols seem to give a better recording of GABAAR-mediated membrane currents. In this protocol, a constantly changing but monitored voltage (at a steady rate) is applied and the current is constantly measured simultaneously36,41. During this protocol, the application of voltage pulses (usually in hyperpolarizing direction) is used to activate the leak current as a result of ohmic responses. Typically, the GABAAR-mediated membrane currents are calculated from the leak current (i.e., the reversal potentials of the leak-subtracted agonist currents)36. The current-voltage associations obtained from this protocol help to provide a better understanding of where the changes are occurring in ion concentrations during an experimental scenario involving GABAA receptor channels. Yelhekar et al.41 used a computational model to show that conclusions on stable ion concentrations from the interpolation method may not be justifiable because the estimated reversal potential from the method may be incorrect.
The average resistance used for most recordings is 5 MΩ. Pipettes with lower resistance of 3-4 MΩ may result in lower series resistance which is preferable for voltage-clamp recordings. However, the pipette's tip will be comparably wider for higher resistance pipettes and, as a consequence, it poses a challenge in the form of difficulty in seal formation and stability36,42. Hence, it is necessary to monitor the GΩ formation to obtain recordings with a considerable reduction in the level of generated noisy data. The robustness of this technique to study the function and activities of KCC2 as adjudged by its suitability and reliability in the measurement of GABA activity has been confirmed by several studies. Previous studies have shown that [Cl–]i remain stable using this technique by measuring the responses of GABAARs (which are capable to gate chloride conductance) to their respective agonists. By implication, continuous electrical access comes with little or no modification of intracellular chloride concentration43,44. Furthermore, this technique facilitates circumvention of artificial changes in membrane potential and EGABA during GABAAR activation (which opens chloride and bicarbonate conductance)45. The aforementioned provides this technique an edge ahead of other types of electrophysiology recordings. However, the limitations of this technique include the following: (1) frequent recording noise may occur due to the tip of the electrode occupying part of the membrane which may reduce the current resolution, and (2) perforation of the membrane with gramicidin usually takes a relatively longer amount of time.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by The Royal Society UK (Grant no. IECNSFC201094), and a Commonwealth Ph.D. Scholarship.
Materials
| 40% acrylamide | Sigma-Aldrich | A2917 | Used to make seperating and stacking gel for SDS-PAGE |
| Ammonium Per Sulfate | Sigma-Aldrich | 248614 | Used to make seperating and stacking gel for SDS-PAGE |
| anti pSPAK | Dundee University | S670B | Used as primary antibody for western blotting |
| anti-KCC2 | Dundee University | S700C | Used as primary antibody for western blotting |
| anti-KCC2 pSer940 | Thermo Fisher Scientific | PA5-95678 | Used as primary antibody for western blotting |
| anti-KCC2 pThr1007 | Dundee University | S961C | Used as primary antibody for western blotting |
| anti-KCC2 pThr906 | Dundee University | S959C | Used as primary antibody for western blotting |
| anti-mouse | Cell Signalling technology | 66002 | Used as secondary antibody for western blotting |
| anti-NKCC1 | Dundee University | S841B | Used as primary antibody for western blotting |
| anti-NKCC1 pThr203/207/212 | Dundee University | S763B | Used as primary antibody for western blotting |
| anti-rabbit | Cell Signalling technology | C29F4 | Used as secondary antibody for western blotting |
| anti-sheep | abcam | ab6900 | Used as secondary antibody for western blotting |
| anti-SPAK | Dundee University | S669D | Used as primary antibody for western blotting |
| anti-β-Tubulin III | Sigma-Aldrich | T8578 | Used as primary antibody for western blotting |
| Benzamine | Merck UK | 135828 | Used as component of lysis buffer |
| Beta-mercaptoethanol | Sigma-Aldrich | M3148 | Used as component of loading buffer and lysis buffer |
| Bradford Coomasie | Thermo Scientific | 1856209 | Used for lysate protein quantification |
| Casting apparatus | Atto | WSE-1165W | Used to run SDS-page electrophoresis |
| Centrifuge | Eppendorf | 5804 | Used in lysate preparation |
| Centrifuge | VWR | MicroStar 17R | Used for spinning samples |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D2650-100ML | Used for cell culture experiment |
| Dried Skimmed Milk | Marvel | N/A | Used to make blocking buffer |
| Dulbecco's Modified Eagle's Medium – high glucose | Sigma-Aldrich | D6429 | Used for cell culture |
| ECL reagent | Perkin Elmer | ORTT755/2655 | Used to develop image for western blotting |
| EDTA | Fisher Scientific | D/0700/53 | Used as component of lysis buffer |
| EGTA | Sigma-Aldrich | e4378 | Used as component of lysis buffer |
| Electrophoresis Power Supply | BioRad | PowerPAC HC | To supply power to run SDS-page electrophoresis |
| Ethanol | ThermoFisher | E/0650DF/17 | Used for preparing sterilized equipments and environment |
| Fetal Bovine Serum - heat inactivated | Merck Life Sciences UK | F9665 | Used for cell culture |
| Fumehood | Walker | A7277 | Used for cell culture |
| Gel Blotting – Whatman | GE Healthcare | 10426981 | Used in western blotting to make transfer sandwich |
| Glycine | Sigma-Aldrich | 15527 | Used to make buffers |
| GraphPad Prism Software | GraphPad Software, Inc., USA | Version 6.0 | Used for plotting graphs and analysing data for western blotting |
| HCl | Acros Organics | 10647282 | Used to make seperating and stacking gel for SDS-PAGE |
| Heating block | Grant | QBT1 | Used to heat WB loading samples |
| HEK293 cells | Merck UK | 12022001-1VL | Cell line for culture experiment |
| ImageJ Software | Wayne Rasband and Contributors; NIH, USA | ImageJ 1.53e | Used to measure band intensities from western blotting images |
| Imaging system | BioRad | ChemiDoc MP | Used to take western blotting images |
| Incubator | LEEC | LEEC precision 190D | Used for cell culture |
| Isopropanol | Honeywell | 24137 | Used in casting gel for electrophoresis |
| L-glutamine solution | Sigma-Aldrich | G7513 | Used for cell culture |
| Lithium dodecyl sulfate (LDS) | Novex | NP0008 | Used as loading buffer for western blotting |
| MEM Non-essential amino acid | Merck Life Sciences UK | M7145 | Used for cell culture |
| Microcentrifuge | Eppendorf | 5418 | Used for preparing lysates for WB |
| Microplate reader | BioRad | iMark | Used for lysate protein concentration readout |
| Microsoft Powerpoint | Microsoft, USA | PowerPoint2016 | Used to edit western blotting images |
| Molecular Weight Marker | BioRad | 1610373 | Used for western blotting |
| N-ethylmaleimide | Thermo Fisher Scientific | 23030 | Used for cell culture experiment |
| Nitrocellulose membrane | Fisher Scientific | 45004091 | Used for western blotting |
| Penicillin-Streptomycin | Gibco | 15140122 | Used for cell culture |
| pH Meter | Mettler Toledo | Seven compact s210 | Used to monitor pH of buffer solutions |
| Phenylmethylsulfonylfluoride (PMSF) | Sigma-Aldrich | P7626 | Used as component of lysis buffer |
| Phosphate Buffer Saline | Sigma-Aldrich | D8537 | Used for cell culture |
| PKCδ pThr505 | Cell Signalling technology | 9374 | Used as primary antibody for western blotting |
| Sepharose Protein G | Generon | PG50-00-0002 | Used for immunoprecipitation |
| Sodium chloride | Sigma-Aldrich | S7653 | Used as component of wash buffer |
| Sodium Chloride | Sigma-Aldrich | S7653 | Used to prepare TBS-T buffer |
| Sodium Dodecyl Sulfate | Sigma-Aldrich | L5750 | Used to make seperating and stacking gel for SDS-PAGE |
| sodium orthovanadate | Sigma-Aldrich | S6508 | Used as component of lysis buffer |
| Sodium Pyruvate | Sigma-Aldrich | S8636 | Used for cell culture |
| sodium-β-glycerophosphate | Merck UK | G9422 | Used as component of lysis buffer |
| Staurosporine (from Streptomyces sp.) | Scientific Laboratory Supplies, UK | S4400-1MG | Used for cell culture experiment |
| Sucrose | Scientifc Laboratory Supplies | S0389 | Used as component of lysis buffer |
| TEMED | Sigma-Aldrich | T7024 | Used to make seperating and stacking gel for SDS-PAGE |
| Transfer Chamber | BioRad | 1658005EDU | Used in western blotting to transfer protein on membrane |
| Tris | Sigma-Aldrich | T6066 | Used to make seperating and stacking gel for SDS-PAGE |
| Triton-X100 | Sigma-Aldrich | T8787 | Used as component of lysis buffer |
| Trypsin-EDTA Solution | Merck Life Sciences UK | T4049 | Used for cell culture |
| Tween-20 | Sigma-Aldrich | P3179 | Used as make TBS-T buffer |
| Vacuum pump | Charles Austen | Dymax 5 | Used for cell culture |
| Vortex | Scientific Industries | K-550-GE | Used in sample preparation |
| Vortex mixer | Scientific Industries Ltd | Vortex-Genie K-550-GE | Used of mixing resolved sample |
| Water bath | Grant Instruments Ltd. (JB Academy) | JBA5 | Used to incubate solutions |
Referencias
- de Los Heros, P., et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+-Cl- co-transporters. Biochemical Journal. 458 (3), 559-573 (2014).
- Heubl, M., et al. GABAA receptor dependent synaptic inhibition rapidly tunes KCC2 activity via the Cl(-)-sensitive WNK1 kinase. Nature Communications. 8 (-), 1776 (2017).
- Schulte, J. T., Wierenga, C. J., Bruining, H. Chloride transporters and GABA polarity in developmental, neurological and psychiatric conditions. Neuroscience & Biobehavioral Reviews. 90, 260-271 (2018).
- Shekarabi, M., et al. WNK Kinase Signaling in Ion Homeostasis and Human Disease. Cell Metabolism. 25 (2), 285-299 (2017).
- Rivera, C., et al. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 397 (6716), 251-255 (1999).
- Kahle, K. T., et al. Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends in Neuroscience. 36 (12), 726-737 (2013).
- Andrews, K., Josiah, S. S., Zhang, J. The Therapeutic Potential of Neuronal K-Cl Co-Transporter KCC2 in Huntington’s Disease and Its Comorbidities. International Journal of Molecular Sciences. 21 (23), 9142 (2020).
- Friedel, P., et al. WNK1-regulated inhibitory phosphorylation of the KCC2 cotransporter maintains the depolarizing action of GABA in immature neurons. Science Signaling. 8 (383), 65 (2015).
- Watanabe, M., et al. Developmentally regulated KCC2 phosphorylation is essential for dynamic GABA-mediated inhibition and survival. Science Signaling. 12 (603), (2019).
- Rinehart, J., et al. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 138 (3), 525-536 (2009).
- Lu, D. C. -. Y., et al. The role of WNK in modulation of KCl cotransport activity in red cells from normal individuals and patients with sickle cell anaemia. Pflügers Archiv-European Journal of Physiology. 471 (11-12), 1539-1549 (2019).
- Huang, H., et al. The WNK-SPAK/OSR1 Kinases and the Cation-Chloride Cotransporters as Therapeutic Targets for Neurological Diseases. Aging and Disease. 10 (3), 626-636 (2019).
- AlAmri, M. A., Kadri, H., Alderwick, L. J., Jeeves, M., Mehellou, Y. The Photosensitising Clinical Agent Verteporfin Is an Inhibitor of SPAK and OSR1 Kinases. Chembiochem. 19 (19), 2072-2080 (2018).
- Zhang, J., et al. Modulation of brain cation-Cl(-) cotransport via the SPAK kinase inhibitor ZT-1a. Nature Communications. 11 (1), 78 (2020).
- Zhang, J., et al. Staurosporine and NEM mainly impair WNK-SPAK/OSR1 mediated phosphorylation of KCC2 and NKCC1. PLoS One. 15 (5), 0232967 (2020).
- Alessi, D. R., et al. The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Science Signaling. 7 (334), 3 (2014).
- Zhang, J., et al. Functional kinomics establishes a critical node of volume-sensitive cation-Cl(-) cotransporter regulation in the mammalian brain. Scientific Reports. 6, 35986 (2016).
- Hartmann, A. M., et al. Opposite effect of membrane raft perturbation on transport activity of KCC2 and NKCC1. Journal of Neurochemistry. 111 (2), 321-331 (2009).
- Pisella, L. I., et al. Impaired regulation of KCC2 phosphorylation leads to neuronal network dysfunction and neurodevelopmental pathology. Science Signaling. 12 (603), (2019).
- Blaesse, P., et al. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. The Journal of Neuroscience. 26 (41), 10407-10419 (2006).
- Medina, I., Pisella, L. I. . Neuronal Chloride Transporters in Health and Disease. , 21-41 (2020).
- Thomas, P., Smart, T. G. HEK293 cell line: a vehicle for the expression of recombinant proteins. Journal of Pharmacological and Toxicological Methods. 51 (3), 187-200 (2005).
- Friedel, P., et al. A Novel View on the Role of Intracellular Tails in Surface Delivery of the Potassium-Chloride Cotransporter KCC2. eNeuro. 4 (4), (2017).
- Lee, Y. -. C., et al. Impact of detergents on membrane protein complex isolation. Journal of Proteome Research. 17 (1), 348-358 (2018).
- Vallée, B., Doudeau, M., Godin, F., Bénédetti, H. Characterization at the Molecular Level using Robust Biochemical Approaches of a New Kinase Protein. JoVE (Journal of Visualized Experiments). (148), e59820 (2019).
- Johansen, K., Svensson, L. . Molecular Diagnosis of Infectious Diseases. , 15-28 (1998).
- Mahmood, T., Yang, P. -. C. Western blot: technique, theory, and trouble shooting. North American Journal of Medical Sciences. 4 (9), 429 (2012).
- Klein, J. D., O’Neill, W. C. Volume-sensitive myosin phosphorylation in vascular endothelial cells: correlation with Na-K-2Cl cotransport. American Journal of Physiology-Cell Physiology. 269 (6), 1524-1531 (1995).
- Hannemann, A., Flatman, P. W. Phosphorylation and transport in the Na-K-2Cl cotransporters, NKCC1 and NKCC2A, compared in HEK-293 cells. PLoS One. 6 (3), 17992 (2011).
- Liu, J., Ma, X., Cooper, G. F., Lu, X. Explicit representation of protein activity states significantly improves causal discovery of protein phosphorylation networks. BMC Bioinformatics. 21 (13), 1-17 (2020).
- Terstappen, G. C. Nonradioactive rubidium ion efflux assay and its applications in drug discovery and development. Assay and Drug Development Technologies. 2 (5), 553-559 (2004).
- Carmosino, M., Rizzo, F., Torretta, S., Procino, G., Svelto, M. High-throughput fluorescent-based NKCC functional assay in adherent epithelial cells. BMC Cell Biology. 14 (1), 1-9 (2013).
- Adragna, N. C., et al. Regulated phosphorylation of the K-Cl cotransporter KCC3 is a molecular switch of intracellular potassium content and cell volume homeostasis. Frontiers in Cellular Neuroscience. 9, 255 (2015).
- Zhang, D., Gopalakrishnan, S. M., Freiberg, G., Surowy, C. S. A thallium transport FLIPR-based assay for the identification of KCC2-positive modulators. Journal of Biomolecular Screening. 15 (2), 177-184 (2010).
- Yu, H. B., Li, M., Wang, W. P., Wang, X. L. High throughput screening technologies for ion channels. Acta Pharmacologica Sinica. 37 (1), 34-43 (2016).
- Hill, C. L., Stephens, G. J. An Introduction to Patch Clamp Recording. Patch Clamp Electrophysiology. , 1-19 (2021).
- Conway, L. C., et al. N-Ethylmaleimide increases KCC2 cotransporter activity by modulating transporter phosphorylation. Journal of Biological Chemistry. 292 (52), 21253-21263 (2017).
- Heigele, S., Sultan, S., Toni, N., Bischofberger, J. Bidirectional GABAergic control of action potential firing in newborn hippocampal granule cells. Nature Neuroscience. 19 (2), 263-270 (2016).
- Moore, Y. E., Deeb, T. Z., Chadchankar, H., Brandon, N. J., Moss, S. J. Potentiating KCC2 activity is sufficient to limit the onset and severity of seizures. Proceedings of the National Academy of Sciences of the United States of America. 115 (40), 10166-10171 (2018).
- Kim, H. R., Rajagopal, L., Meltzer, H. Y., Martina, M. Depolarizing GABAA current in the prefrontal cortex is linked with cognitive impairment in a mouse model relevant for schizophrenia. Science Advances. 7 (14), 5032 (2021).
- Yelhekar, T. D., Druzin, M., Karlsson, U., Blomqvist, E., Johansson, S. How to properly measure a current-voltage relation?-interpolation vs. ramp methods applied to studies of GABAA receptors. Frontiers in Cellular Neuroscience. 10, 10 (2016).
- Ishibashi, H., Moorhouse, A. J., Nabekura, J. Perforated whole-cell patch-clamp technique: a user’s guide. Patch Clamp Techniques. , 71-83 (2012).
- Ebihara, S., Shirato, K., Harata, N., Akaike, N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. The Journal of Physiology. 484 (1), 77-86 (1995).
- Kyrozis, A., Reichling, D. B. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. Journal of Neuroscience Methods. 57 (1), 27-35 (1995).
- Lamsa, K., Palva, J. M., Ruusuvuori, E., Kaila, K., Taira, T. Synaptic GABAA activation inhibits AMPA-kainate receptor-mediated bursting in the newborn (P0-P2) rat hippocampus. Journal of Neurophysiology. 83 (1), 359-366 (2000).

