Culturing Lymphocytes in Simulated Microgravity Using a Rotary Cell Culture System
Summary
This is a step-by-step guide for using a commercially available rotary cell culture system to culture lymphocytes in simulated microgravity using specialized disposable culture vessels. This culturing method may be applied to any suspension-type cell culture.
Abstract
Given the current limitations of conducting biological research in space, a few options exist for subjecting cell culture to simulated microgravity (SMG) on Earth. These options vary in their methods, principles, and suitability for use with suspension cell culture. Here, a cell culture method is described for subjecting lymphocytes to simulated microgravity using a commercially available rotary cell culture system, also known as a 2D clinostat or a rotating wall vessel (RWV) device. This cell culture method utilizes the principle of time-averaged gravity vector nullification to simulate microgravity by rotating the cells on a horizontal axis. The cells cultured in this system can be harvested and utilized in many different experimental assays to assess the effects of simulated microgravity on cellular function and physiology. The culturing technique may vary slightly depending on the cell type or line that is used, but the method described here may be applied to any suspension-type cell culture.
Introduction
Spaceflight has been shown to impact many aspects of human physiology, including the immune system. Many studies have demonstrated evidence of immune dysregulation as a result of spaceflight in vivo and exposure to simulated microgravity (SMG) in vitro1,2,3,4. One major aspect of the space environment that impacts human physiology is microgravity. Microgravity refers to the "weightlessness" experienced due to low gravitational forces in the space environment5. As humanity prepares for longer space missions to the Moon and Mars, more research needs to be conducted to mitigate serious health risks in astronauts.
Real microgravity conditions for scientific research can be achieved in space onboard the International Space Station (ISS) or in nanosatellites launched into orbit; however, these options can be incredibly costly and complex to orchestrate. Given the current limitations of conducting biological research in space, several options exist for inducing real microgravity and SMG on Earth. Large-scale operations exist that can produce short periods of real microgravity on Earth, including drop towers, parabolic flight, and sounding rockets. However, these methods are not overly suitable for studying the effects of microgravity on biological systems, largely due to their short periods of microgravity treatment (i.e., seconds to 20 min). These methods are discussed in greater detail elsewhere5,6. Options that are suitable for biological cell culture include small-scale devices such as 2D clinostats or rotating wall vessel (RWV) devices and 3D clinostats or random positioning machines (RPM). These devices can be set up inside cell culture incubators maintained at 37 °C and 5% CO2, and they rotate the cell culture either on a horizontal axis (2D) or on two perpendicular axes (3D)5. However, it is important to emphasize that these culturing methods produce SMG as opposed to real microgravity, which is most feasibly attained in space for biological research contexts.
The goal of the current paper is to outline the steps for subjecting lymphocytes to SMG using a commercially available RWV device (Table of Materials), which falls under the 2D clinostat classification. While there is a general protocol available from the manufacturer for operating this device, the current article aims to cover the troubleshooting and optimization steps in more detail. This article also covers the theory behind how this device works to produce SMG in suspension cell culture, specifically with lymphocytes. In this context, suspension cell culture refers to cells growing freely in supplemented culture media, without adhering to any additional scaffolding. Many cell types are grown in suspension cell culture, including lymphocytes. Lymphocytes are cells of the immune system, including T, B, and Natural Killer (NK) cells, that reside in lymphoid organs and the bloodstream7.
The RWV 2D clinostat described here operates on the principle of time-averaged gravity vector nullification5,6,8,9, whereby the gravity vector is randomized through rotation of the cell culture on a horizontal axis. This is achieved by matching the rotational velocity of the culture vessel to the sedimentation velocity of the cells. As long as the rotational velocity of the culture vessel is matched well to the sedimentation velocity of the cells, the cells are maintained in free-fall and unable to sediment, as experienced in the space environment. After an initial speed-up phase, the media in the culture vessel eventually reaches "solid body rotation" over time. This horizontal rotation also induces laminar flow in the cell culture vessel. This creates a "low shear" environment, given that the shear stress induced on the cells by laminar flow is much less than that of turbulent flow. However, given that the clinostat is not a perfect system, there are some small, laminar fluid motions introduced, which inflict minimal shear stress on the cells. As such, the cells suspended in the media get dragged along by this flow during rotation. During horizontal rotation, the gravity vector acts on the cells and brings them into an oscillating trajectory, as visualized in Figure 1. Another small source of shear stress is caused by the cells "falling" through the media, causing laminar flow around the cells. As the culture vessel rotates on a horizontal axis, the gravity vector experienced by the cells rotates as well. Over time, this rotating gravity vector averages to approach zero; this phenomenon is called time-averaged gravity vector nullification and induces a state of SMG5,6,8,9. This device has been used to study the effects of SMG on many types of cells, some of which are covered in references10,11,12. More examples can be found on the device manufacturer's website.
This RWV device uses specialized "high aspect ratio vessels" (HARVs) available through the device manufacturer. These HARVs hold 10 mL of cell culture each; however, 50 mL HARVs are also available. Either 10 mL or 50 mL HARVs can be used depending on how many cells are needed to complete any downstream experimental assays, which is outlined further in the discussion section. The HARVs are made of polycarbonate and include a silicone oxygenation membrane to allow for gas exchange during cell culture. This maintains the pH of the cell media and allows for efficient cellular respiration. There is a main fill port and two capped syringe ports on the face of the vessel (Figure 2A). After loading the cell culture through the main fill port, two syringes are loaded onto the vessel to assist with bubble removal. When using the 10 mL vessels, two 3 mL syringes work well. One syringe is attached to the device empty, with the syringe completely depressed, and the other is attached filled with 3 mL of cell culture (Figure 2E). These are used in combination to remove bubbles from the vessel, which is important for maintaining the SMG treatment. In general, it is advised to set up two negative controls, which can be referred to as the "Flask" control and the "1G" control. The "Flask" control corresponds to cells that are grown in a standard T25 suspension cell culture flask. The 1G control corresponds to cells that are grown in the specialized 10 mL culture vessel, which is simply placed in the incubator (i.e., without being subjected to the SMG treatment). Please see the Discussion section for more details on controls.
The method described here is appropriate for any researcher looking to study the effects of SMG on lymphocytes, with a specific focus on NK cells by using the NK92 cell line13. The results from these studies may help us better understand and mitigate the adverse effects of spaceflight on the human immune system.
Protocol
NOTE: The following steps should be completed inside a sterile biological safety cabinet.
1. Preparation of vessels for cell culture
- Take the culture vessels out of the plastic packaging. Label each vessel on the rim according to what cell type/line is being used, whether it is the control (1G) or treatment (SMG), and any other relevant information.
- Stabilize the vessel and open the fill port carefully, without touching the fill port cap peg/O-ring. Place the cap on an ethanol pad, with the peg/O-ring facing up, while the vessel is being filled. Do not remove the caps from the syringe ports.
NOTE: If the peg/O-ring is accidentally touched, spray the cap peg with 70% ethanol and let it sit (facing upward) for a few moments while filling the vessel. When ready to close the fill port again, pick up the cap with a fresh paper wipe to thoroughly dry the cap before placing it back on the vessel. - Load the vessel with 10 mL of the appropriate complete media for the cell culture using a sterile serological pipette, and carefully place the cap back on the fill port.
NOTE: Here, the NK92 NK cell line was used, which was cultured in GM1 media, as previously validated in a clinical trial using NK92 cells as a cell therapy14. The culture media used depends on the cell line or cell type being cultured. Please check the supplier's website for the correct media recipe for the cell line or cell type being used. - Ensure that the syringe port caps and fill port are securely closed, and place the filled vessels in a 37 °C, 5% CO2 incubator while carrying out the subsequent steps to prime the vessels for cell culture.
NOTE: This priming step helps to minimize large bubble formation to make expelling bubbles easier later in Step 6.
2. Preparation of stock cell culture for the SMG treatment setup
- When not in use, store the cells of interest at a temperature lower than -130 °C or, ideally, in a liquid nitrogen vapor phase.
- At least 1 week before setting up the planned treatment, thaw the cells and culture them using the appropriate cell culture media recipe found on the supplier's website. Plan how many cells are needed to set up the treatment (details below in the subsequent steps) and ensure to start the cultures early enough to allow for sufficient proliferation and adaptation to culture.
- Store the cell culture in a 37 °C, 5% CO2 incubator. Further details on standard cell culture practice are covered elsewhere15.
- On the day of the planned SMG treatment setup, determine the starting concentration and viability of the initial cell culture.
- Use the preferred method for determining the concentration of the initial cell culture (e.g., hemocytometer, ViCell, flow cytometry, etc.). Take note of the viable cell concentration (cells per mL) and viability (%) of the cells, ensuring that the viability is ideally greater than 85%. A protocol for using a hemocytometer can be found elsewhere16.
- Determine the appropriate cell seeding density/concentration of the cell culture for SMG treatment by considering the optimal cell concentration range of the particular cell type/line, their doubling time, and the length of SMG treatment. Please refer to the Discussion section for further elaboration.
NOTE: From experience, the optimal concentration range for NK92 cells is between 0.3 x 106– 1.2 x 106 cells/mL. Generally, these cells have a doubling time of 48-72 h and are fed every 2-3 days as such. Given that the treatment length was 72 h, the cells were seeded at the lower end of their optimal range at 0.4-0.5 x 106 cells/mL. The supplier also recommends this seeding density when starting the initial cell culture from a fresh vial of NK92 cells. - Determine the volume of stock cell culture that is needed to set up the treatment and the controls.
NOTE: 10 mL of cell culture is needed per experimental group. If three experimental groups are set up (i.e., one treatment: "SMG", and two negative controls: "Flask" and "1G"), another 10 mL of cell culture is sufficient to aliquot into a 15 mL tube used to load the HARV syringes. This is 40 mL in total, which may fluctuate depending on the specific experimental design. - Determine the number of cells needed to set up the treatment.
- Calculate the total number of cells needed by multiplying the chosen seeding density (cells/mL) by the total volume needed (mL).
NOTE: From experience with NK92, 40 mL of stock cell culture is prepared as detailed above at a seeding concentration of 0.4-0.5 x 106 cells/mL. As such, 16-20 x 106 NK92 cells are needed to complete the typical treatment setup. For the NK92 cell line, using slightly more cells is better than slightly fewer cells to maintain high viability throughout the treatment.
- Calculate the total number of cells needed by multiplying the chosen seeding density (cells/mL) by the total volume needed (mL).
- Determine the volume of initial cell culture to spin down.
- Given that cell concentration is represented as cells/mL, divide the total number of cells (cells) needed for the experiment by the measured starting concentration of the cell culture (cells/mL).
NOTE: For example, if 16 x 106 cells are needed and the starting concentration of the culture is 0.8 x 106 cells/mL, 20 mL of the culture should be spun down.
- Given that cell concentration is represented as cells/mL, divide the total number of cells (cells) needed for the experiment by the measured starting concentration of the cell culture (cells/mL).
- Make the final stock cell culture.
- Centrifuge the appropriate number of cells at 300 x g for 8 min in a 50 mL conical tube.
- Carefully pour off the supernatant into a waste container, and gently flick the tube to resuspend the pellet in the small leftover volume.
- Resuspend the cells in the appropriate volume of warmed (37 °C) complete media to bring the culture to the desired seeding density. This volume was calculated in Step 2.6.
- Securely cap the 50 mL tube, and gently invert it a few times to thoroughly mix the cell suspension.
NOTE: If more than 50 mL are needed to set up the full experiment, it is best to centrifuge the total number of cells needed in a 50 mL conical tube and resuspend this in half the volume needed. Then, mix well and make a 1:2 dilution in a separate 50 mL conical tube. For example, if 100 mL is needed at 0.4 x 106 cells/mL, spin down 40 x 106 cells in a 50 mL tube, resuspend in 50 mL of media, transfer 25 mL to another 50 mL tube, and then top up both with 25 mL of fresh media.
3. Loading vessels with stock cell culture
- Retrieve the media-primed vessels from the incubator.
- If a "Flask" control is being used, load a T25 suspension culture flask with 10 mL of cell culture from the stock prepared above. Also, add 10 mL of stock cell culture to a separate 15 mL conical tube, which will be used to load the syringes.
- Unscrew the syringe port caps to remove them from the vessel and ensure the stopcocks are in the open position (Figure 2C).
- Stabilize the vessel and open the fill port carefully, without touching the fill port cap peg/O-ring. Place the cap on an ethanol pad, with the peg/O-ring facing up, while the vessel is being filled. Carefully unscrew the caps from the syringe ports.
- Ensure that the stopcocks are in the open position (Figure 2C). Carefully pour out the media from the vessel into a waste container, remove the media using a serological pipette, or aspirate it using a sterile glass Pasteur pipette attached to the vacuum system, without touching the oxygenation membrane.
- Tightly close the 50 mL conical tube that contains the stock cell culture and gently invert it a few times to thoroughly mix the contents.
- Draw up 10 mL of cell culture stock from the 50 mL tube with a fresh sterile serological pipette. Pick up the vessel and tilt it so that the fill port is toward the top and then carefully dispense the cell culture stock into the vessel through the fill port.
NOTE: Be careful and watch the volume so that the culture does not spill through the syringe ports while tilting the vessel. - Aim to fill the vessel right to the top of the lip of the fill port without spilling; tilt the vessel back down as it is being filled to avoid spilling. Be careful not to touch the oxygenation membrane with the pipette as it is very fragile.
NOTE: The vessel filling process may take some practice; be patient and go slowly. If a bubble film forms at the opening of the fill port, this will interfere with loading the vessel. If this happens, gently hit the vessel to break the seal created by the bubble film. - Once the vessel is loaded, carefully place the fill port cap back on, ensuring not to touch the peg/O-ring.
- Place on the syringes.
NOTE: There are two syringe ports on the face of the vessel (Figure 2A). One will hold an empty syringe, and the other will hold a syringe full of cell culture (Figure 2E).- First, attach the 3 mL empty syringe to one of the syringe ports, ensuring that the syringe is fully depressed.
NOTE: Pump the syringes a few times before attaching them to the vessel as they are a bit tight at first. Do this inside of the biological safety cabinet to maintain sterility. - Tightly close the 15 mL conical tube with 10 mL of aliquoted cell culture and gently invert it a few times to thoroughly mix the contents.
- Next, carefully semi-submerge the second 3 mL syringe in the cell culture and draw up some culture. To minimize the air bubble in the top of the syringe, completely dispense this back into the tube, and then draw up 3 mL of the culture.
- Attach the filled syringe to the remaining syringe port, and carefully sanitize the syringes and around the fill port with an ethanol pad. Do not get ethanol on the oxygenation membrane. Now the vessel is ready for being rid of bubbles.
NOTE: The second filled syringe for the second vessel is more difficult to load given the depth of the 15 mL tube. It helps to tilt the tube while filling the syringe to maintain the seal between the syringe being filled and the cell culture. - Repeat Steps 3.3-3.10 for the remaining vessels. If there is a slightly insufficient amount of cell culture left in the 50 mL conical tube for the last vessel (e.g., 9.5 mL instead of 10 mL), recover the remaining amount from the 15 mL conical tube used to load the syringes.
NOTE: The following steps do not need to take place within a sterile biological safety cabinet, as the vessel is now a closed, sterile system.
- First, attach the 3 mL empty syringe to one of the syringe ports, ensuring that the syringe is fully depressed.
4. Removing bubbles from the vessels
NOTE: Bubbles are inevitable within this setup and must be consistently removed throughout the treatment (Figure 3). Please refer to the Discussion section for more details on this.
- Ensure that the syringe port stopcocks are in the closed position (Figure 2D) to limit the migration of cells and bubbles from the syringes into the vessels. This is especially important later during the treatment (discussed below). To collect the initial bubbles, flip the vessel upside down and hit the side of it a few times.
- Quickly flip the vessel back to face upward and then on a slight angle facing away so that the bubbles all float to the upward side of the vessel (Figure 3B).
- Maneuver the bubbles under the port with the empty syringe and watch them start to enter up into the port. Open both of the syringe port stopcocks (Figure 2C).
- Gently hit the vessel to encourage the bubbles to float up into the empty syringe. Slowly suck up the larger bubbles with the empty syringe, while carefully depressing the full syringe, to maintain the pressure in the vessel so that the oxygenation membrane does not burst.
NOTE: Larger bubbles will need to be sucked up, whereas small bubbles and microbubbles can be encouraged to float up into the syringe by maneuvering them into the syringe ports and gently hitting the vessel. After a few hours of treatment, it is highly important to minimize the "cross-contamination" between the cells in the syringes and the cells in the culture vessel. This is because the cells in the syringes do not receive the same amount of oxygenation or exposure to SMG as the cells in the vessel do. - Repeat Steps 4.2-4.4 a few times to ensure all bubbles are removed. Remove all bubbles including microbubbles from the vessels.
NOTE: The bubbles can be easily seen through the oxygenation membrane (back of the vessel) when the vessel is aimed at a light source (window, light, etc.). When carrying out Steps 4.2 and 4.3, look at the bubbles moving from the back of the vessel to help visualize the harder-to-see bubbles. - When the bubbles have been effectively removed, close the syringe port stopcocks. Keep the syringes on during treatment to allow for subsequent bubble removal, ensuring that the volume in both syringes is approximately equal.
5. Attaching the vessel to the rotating base
- Carefully wipe down the surface of the rotating base and ribbon cable with 70% ethanol.
- Ensure that the included ribbon cable is attached to the power supply. Place the rotating base in the incubator and attach the ribbon cable to the base. Ensure that the power supply is kept near but outside of the incubator. Figure 4 depicts the rotating base and power supply for context.
- Attach the SMG treatment vessel by lining up the vessel threads to the rotating peg and gently turning the rotating peg on the base in a counterclockwise direction. Ensure the vessel is attached securely. Ensure that the incubator maintains close to 100% humidity by filling the water tray with sufficient autoclaved, reverse osmosis (RO) water.
- Choose an appropriate rotation speed (rpm). Adjust the rotation speed to match the sedimentation velocity of the cells, such that the cells do not "fall through the media" at all but end up rotating in a small orbital path. This phenomenon is visualized as a schematic diagram in Figure 1.
NOTE: The company recommends starting the rotation at 8-10 rpm for lymphocytes. In this case, NK92 cells were rotated at 11 rpm, as demonstrated in a previous paper17. Depending on the cell size, the rpm should be increased for larger cells and decreased for smaller cells. The same pattern applies if the cells used tend to clump during culturing. Please see the Discussion section for further details on this.
6. Treatment
- Choose an appropriate treatment length for the research application, which may depend on what parameters of cell function/physiology are being assayed. Please see the Discussion section for further details on this.
NOTE: In this case, NK92 cells were subjected to a 72 h SMG treatment, based on results from previous studies18,19. Bubbles will inevitably form throughout the treatment and will need to be removed. The rotation can be briefly stopped and restarted to facilitate this. See Step 7.1 for how to safely remove the SMG vessel. - On the first day of setting up treatment, check for bubble formation every few hours by repeating Steps 4.2-4.6. After the first day, check the vessels as necessary (at least once per day), repeating Steps 4.2-4.6, until the end of the treatment.
7. Harvesting cells from the vessels
- Once the planned treatment length has elapsed, stop the rotation, and disassemble the apparatus. Remove the SMG treatment vessel by gently holding the vessel stationary while turning the rotating peg of the device in a clockwise direction.
- Bring the control flask, control 1G vessel, and treated SMG vessel into a sterile biological safety cabinet.
- Take each vessel (1G and SMG only; not the Flask) and flip it upside down so it faces downward and gently hit it to bring all the cells into suspension. Then, turn the vessel on its side with the fill port toward the bottom and hit the vessel again to encourage the cells toward the fill port for efficient aspiration.
- Handle each vessel individually. Unscrew the syringes from the two ports and dispose of them into the biohazard waste. Open the stopcocks on the syringe ports.
- Carefully remove the fill port cap and draw up the contents using a sterile 10 mL serological pipette, tilting the vessel as it is being emptied. Dispense the contents of the "Flask", "1G", and "SMG" groups into individually labeled 15 mL conical tubes.
- Close each tube and gently invert them a few times to ensure they are properly mixed. Use the preferred method for determining the concentration and viability of the resulting cell culture to prepare for use within subsequent experimental assays.
Representative Results
This culturing method is considered successful if 1) the proliferation of the cells is approximately consistent across the control groups (and ideally all experimental groups), 2) the proliferation is appropriate given the seeding density, length of treatment, and the doubling time of the cell type/line, and 3) the viability of the harvested cells is 85% or higher (Table 1). Ideally, the resulting cells should be as healthy as they would be in standard cell culture, especially for use in subsequent experiments and assays (i.e., viability 85% or higher). This culturing method is considered unsuccessful if the opposite is true, whereby the resulting cells either die, differ substantially in proliferation across the control groups, or have suboptimal viability around 70% or lower (Table 1). Proliferation in the SMG treatment group may or may not differ compared to the controls, depending on how the SMG treatment affects the cellular physiology. However, this has not been an issue to date, and the cell proliferation was approximately equal across the control and treatment groups (Table 1). As mentioned previously, these parameters are important for the success of downstream assays and experiments, and the resulting cells from the two control groups should perform relatively similarly.
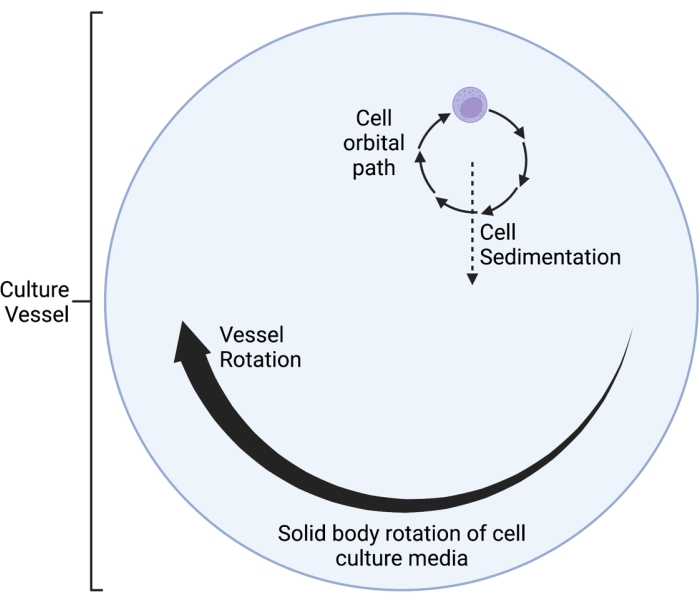
Figure 1: Schematic diagram of the localized orbital path of the cells cultured within the simulated microgravity (SMG) vessel during operation. The RWV 2D clinostat described here operates on the principle of time-averaged gravity vector nullification5,6,8,9, whereby the gravity vector is randomized through rotation of the cell culture on a horizontal axis. This is achieved by matching the rotational velocity of the culture vessel to the sedimentation velocity of the cells. After an initial speed-up phase, the media in the culture vessel eventually reaches "solid body rotation" over time. This horizontal rotation also induces laminar flow in the cell culture vessel. This creates a "low shear" environment, given that the shear stress induced on the cells by laminar flow is much less than that of turbulent flow. However, given that the clinostat is not a perfect system, there are some small, laminar fluid motions introduced, which inflict minimal shear stress on the cells. As such, the cells suspended in the media get dragged along by this flow during rotation. During horizontal rotation, the gravity vector acts on the cells and brings them into an oscillating trajectory, which is visualized here. As the culture vessel rotates on a horizontal axis, the gravity vector experienced by the cells rotates as well. Over time, this rotating gravity vector averages to approach zero; this phenomenon is called "time-averaged gravity vector nullification," and induces a state of SMG5,6,8,9. This Figure has been modified from Castro et al., 201120. Created with BioRender.com. Please click here to view a larger version of this figure.
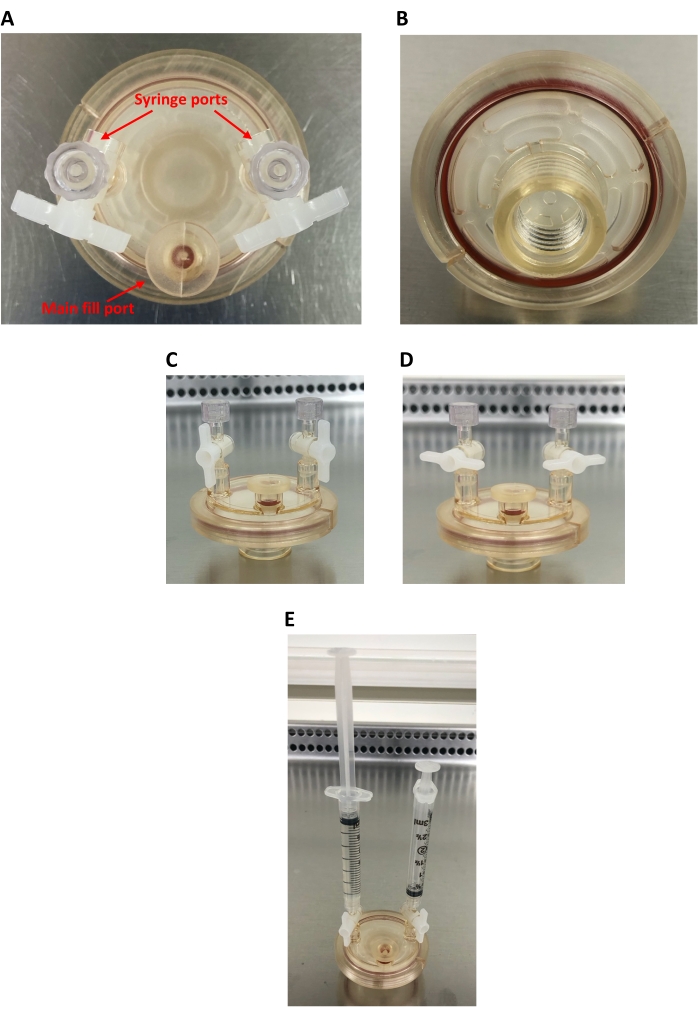
Figure 2: 10 mL high aspect ratio vessel (HARV). (A) Bird's-eye view of the HARV, showing the main fill port and two syringe ports. (B) Back of the HARV showing the screw-on port for connecting the HARV to the rotary base and the oxygenation membrane. (C) Side view of the HARV showing open syringe ports (capped). (D) Side view of the HARV showing closed syringe ports (capped). (E) Side view of the HARV showing the two 3 mL syringes attached; the left syringe is filled with cell culture and the right syringe is empty. Please click here to view a larger version of this figure.
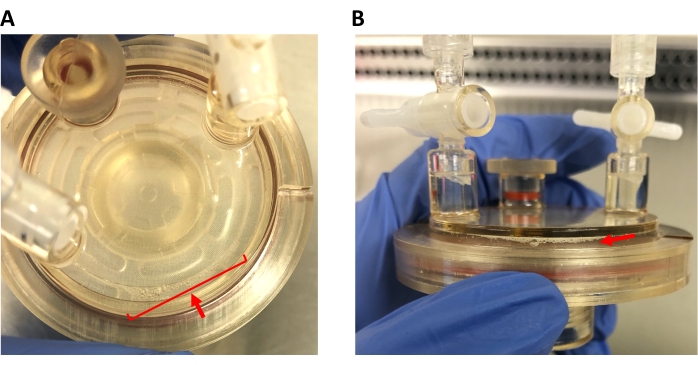
Figure 3: Bubbles in the HARV. (A) Bird's-eye view of the HARV, showing bubbles to be eliminated from the cell culture. (B) Side view of the HARV, showing the same bubbles. Note how the bubbles vary in size; microbubbles must be removed as well. Please click here to view a larger version of this figure.

Figure 4: Rotating base and power supply of the RWV device. (A) Front view of the rotating base, showing four rotating pegs that can accommodate up to four culture vessels. (B) Back view of the rotating base, showing the input for the ribbon cable (not pictured) that links the base and the power supply. (C) Front view of the power supply kept on top of the incubator. Note the on/off switch on the left and the rpm adjustment dial on the right. The power supply is plugged into the nearest outlet (usually on the back of the incubator) and includes an input for the ribbon cable to connect to the rotating base. The power supply stays outside of the incubator. The base is placed inside the incubator (37 °C, 5% CO2) during operation, and the ribbon cable is fed through the incubator door and connected to the power supply. The ribbon cable does not interfere with the incubator seal. When the rotating base is not in use, it should be kept outside of the incubator, safely stored on a lab bench or shelf. Refer to the Table of Materials for the details of the commercial device used. Please click here to view a larger version of this figure.
| # | Starting Viability | Seeding Density cells/mL | End Viability (F) | End Viability (1G) | End Viability (SMG) | End Conc (F) cells/mL | End Conc (1G) cells/mL | End Conc (SMG) cells/mL | Notes | |||||
| Suboptimal starting viability and seeding density (negative outcome) | 1 | 79% | 0.2 x 106 | 67% | 60% | 60% | 0.10 x 106 | 0.075 x 106 | 0.071 x 106 | Low seeding density and suboptimal starting viability led to cell death over the course of the treatment period | ||||
| 2 | 73% | 0.2 x 106 | 43% | 63% | 70% | 0.071 x 106 | 0.081 x 106 | 0.085 x 106 | ||||||
| Optimal starting viability and seeding density (positive outcome) | 3 | 93% | 0.4 x 106 | 93% | 93% | 96% | 1.2 x 106 | 1.1 x 106 | 1.5 x 106 | Appropriate seeding density and optimal starting viability led to healthy cell growth and viability throughout treatment | ||||
| 4 | 92% | 0.4 x 106 | 92% | 92% | 94% | 0.81 x 106 | 0.80 x 106 | 0.70 x 106 | ||||||
Table 1: Comparison chart showing unsuccessful and successful simulated microgravity (SMG) treatments. NK92 starting viability and seeding densities were compared to the resulting end viability and end concentrations after a 72 h SMG treatment in a 37 °C cell culture incubator supplemented with 5% CO2. Two instances of negative outcomes and two instances of positive outcomes were compared. For comparison, note that the optimal concentration range of the NK92 cell line used was between 0.3 x 106 cells/mL and 1.2 x 106 cells/mL, with a doubling time of around 2-3 days.
Discussion
As humanity prepares for longer space missions to the Moon and Mars, more research needs to be conducted to mitigate serious health risks in astronauts. One major aspect of the space environment that impacts human physiology is microgravity. Here, a cell culture method has been described for subjecting lymphocytes to SMG using a commercially available rotary cell culture system.
This protocol contains a few critical steps that may need to be optimized depending on the cell type or line that is used. These include 1) choosing an appropriate seeding density depending on the cells' doubling time and length of SMG treatment, and 2) determining an optimal treatment length, rotation speed, and appropriate controls. Choosing a seeding density that is in the mid-range of appropriate cell concentrations for the cell type or line that is being studied should be sufficient. However, choosing a seeding density that is too low may lead to low cell proliferation and viability (Table 1), and choosing a density that is too high may lead to premature nutrient depletion and low cell viability. The chosen seeding density also depends on the doubling time of the cells that are being studied; cells with a shorter doubling time may be seeded at a lower density, and those with a longer doubling time may need to be seeded at a higher density. The seeding density and length of the treatment also depend on how many cells are needed to complete the subsequent experimental assays. From experience, seeding highly viable (90%+) NK92 cells at 0.4-0.5 x 106 cells/mL in 10 mL vessels (i.e., 4-5 million cells per experimental group; optimal range for the cell line = 0.3 x 106-1.2 x 106 cells/mL, doubling time = 2-3 days) and treating them for 72 h has yielded roughly 8-15 million cells (Table 1). As such, the 10 mL vessels were appropriate for harvesting cells for both functional assays (3 x 106 cells) and collecting cells for qPCR (1 x 106 cells) and western blot (2 x 106-6 x 106 cells). Supernatants can also be collected for analysis of secretory components. However, 50 mL vessels are also available and can be used when greater cell yield is required. When using 50 mL vessels, larger syringes also need to be used.
Determining an appropriate treatment length will also depend on the cell type/line that is used. If previous studies exist, they should be referred to in order to choose an appropriate treatment length to start with. A few studies have used RWV devices to culture NK cells, and are referenced here17,18,19. From there, the treatment outcomes or how well the cells proliferated and their viability, and their performance in subsequent experimental assays, should be examined. It may be possible to extend the treatment length past 72 h by removing the cell culture from the vessels into a tube, centrifuging and then resuspending the cells in 10 mL of warm, fresh complete culture media, replacing them into the vessels, and restarting rotation. However, this may introduce confounds due to exposure to hypergravity through centrifugation, and splitting/diluting the cells may be necessary to ensure the cells are kept within their optimal concentration range. If any stimulatory molecules (e.g., LPS, cytokines, etc.) are to be used, it is recommended that these are added at an appropriate concentration to the complete media recipe before setting up the SMG treatment.
Setting an appropriate rotation speed (rpm) is also key to maintaining the simulated microgravity treatment. The company recommends starting with a rotation speed between 8 and 10 rpm when culturing lymphocytes. From experience, a speed of 11 rpm has worked well to ensure that NK92 cells are kept in suspension and has been used in a past NK cell study17. Depending on the growth patterns of the cell type/line being used, the rotation speed may need to be increased to account for cell clumping. This would lead to increased sedimentation of the cells due to increased mass. For an optimal SMG treatment, the rotation speed of the culture vessel must be adjusted to match the sedimentation velocity of the cells5,6,8,9. In other words, cells or cell clumps should not be seen falling through the media, and they should remain relatively stationary.
In this context, it is good practice to try two negative controls by comparing cells grown in a standard T25 culture flask ("Flask") and cells grown in the specialized HARV but not subjected to SMG (i.e., just placed in the incubator; "1G"). Ideally, the cell performance and outcomes in downstream experimental assays between the two negative controls should be comparable. Any inconsistencies should be noted. For most assays, the best comparison is likely between the "1G" control and SMG treatment; however, including both the "Flask" and "1G" controls may be beneficial for sufficient comparison and initial optimization.
The major limitations of this protocol include 1) bubble formation during cell culture, 2) the extent of SMG, and 3) the possible duration of treatment. It is crucial to monitor bubble formation throughout the SMG treatment. Even minuscule microbubbles can accumulate, grow, and lead to the formation of highly disruptive larger bubbles. These larger bubbles interrupt the low-shear fluid dynamics within the culture vessel, causing increased turbulence as the fluid flow is deflected around the bubble21. Ultimately, this completely disrupts the SMG condition. This phenomenon is discussed at length and visualized by Phelan et al21. Additionally, it is important to keep in mind that this device produces SMG and not real microgravity as experienced onboard the ISS6. Nonetheless, studies have shown similar effects of SMG produced by this device compared to effects of real microgravity from studies performed on the ISS1,5,6.
Alternative methods for subjecting cell culture to SMG do exist. These include the use of 3D clinostats or Random Positioning Machines (RPM) and diamagnetic levitation. 3D clinostats rotate cell culture on two perpendicular axes at the same velocity, while RPMs rotate on two perpendicular axes, whereby both the velocity and directionality of rotation are randomized5,6. Therefore, compared to 2D clinostats or RWV devices, RPMs are more complex, which introduces several benefits and drawbacks. Firstly, the degree of microgravity that can be achieved in an RPM can be modulated to simulate partial gravity, such as that experienced on the Moon (0.16 g) and Mars (0.33 g)6. However, the added complexity of randomized rotational directions and velocities may introduce jerk motion and accelerative forces, especially toward the outer areas of the culture vessel, potentially leading to confounds in the data. Diamagnetic levitation exposes samples to strong repulsive magnetic fields to counteract the weight of water in biological samples, as a way of counteracting gravity. However, the strong magnetic field generated to do so may also negatively impact the cells, therefore introducing confounds to the data5,6. These methods are discussed in more detail elsewhere5,6.
In conclusion, the commercially available rotary cell culture system discussed here is a relatively easy-to-use, accessible platform for scientists looking to study the effects of SMG on lymphocytes. While there are limitations to this cell culture method, it remains a viable option for culturing lymphocytes and potentially other suspension cell cultures in simulated microgravity.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work is supported by the Canadian Space Agency (CSA), research grant (17ILSRA3, Immuno Profile). Authors would like to acknowledge and thank Dr. Roxanne Fournier (University of Toronto), Dr. Randal Gregg (Lincoln Memorial University), and Preteesh Mylabathula (University of Arizona) for their help with the initial troubleshooting of this protocol.
Materials
| Disposible High Aspect Ratio Vessel (HARV) (10 mL) | Synthecon | D-410 | Gamma sterilized culture vessels (4/box) |
| Luer-Lok tip syringes (3 mL) | BD | 309657 | For attaching to the 10 mL HARVs |
| NK92 Cell-line | ATCC | CRL-2407 | |
| Rotary Cell Culture System (RCCS) | Synthecon | RCCS-4D | Rotating wall vessel device; 2D clinostat |
| Sarsedt 15 mL conical tubes | Fisher Scientific | 50-809-220 | |
| Sarsedt 50 mL conical tubes | Fisher Scientific | 50-809-218 | |
| Sarsedt sterile serological pipettes | Fisher Scientific | 86.1254.001 | |
| T25 suspension culture flasks | Sarsedt | 83.3910.502 | For flask control |
Referencias
- ElGindi, M., et al. May the force be with you (or not): the immune system under microgravity. Cells. 10 (8), 1941 (2021).
- Choukèr, A., Ullrich, O. . The Immune System in Space: Are we Prepared. , (2016).
- Crucian, B. E., et al. Immune system dysregulation during spaceflight: potential countermeasures for deep space exploration missions. Frontiers in Immunology. 9, 1437 (2018).
- Crucian, B. E., et al. Countermeasures-based improvements in stress, immune system dysregulation and latent herpesvirus reactivation onboard the International Space Station – relevance for deep space missions and terrestrial medicine. Neuroscience & Biobehavioral Reviews. 115, 68-76 (2020).
- Herranz, R., et al. Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology. 13 (1), 1-17 (2013).
- Ferranti, F., Del Bianco, M., Pacelli, C. Advantages and limitations of current microgravity platforms for space biology research. Applied Sciences. 11 (1), 68 (2020).
- Murphy, K., Weaver, C. . Janeway’s Immunobiology 9th Edition. , (2016).
- Dedolph, R. R., Dipert, M. H. The physical basis of gravity stimulus nullification by clinostat rotation. Plant Physiology. 47 (6), 756-764 (1971).
- Hammond, T. G., Hammond, J. M. Optimized suspension culture: the rotating-wall vessel. American Journal of Physiology-Renal Physiology. 281 (1), 12-25 (2001).
- Crabbé, A. Transcriptional and proteomic responses of Pseudomonas aeruginosa PAO1 to spaceflight conditions involve Hfq regulation and reveal a role for oxygen. Applied and Environmental Microbiology. 77 (4), 1221-1230 (2011).
- Ulbrich, C., et al. The impact of simulated and real microgravity on bone cells and mesenchymal stem cells. BioMed Research International. 2014, 1-15 (2014).
- Martinez, E. M., Yoshida, M. C., Candelario, T. L. T., Hughes-Fulford, M. Spaceflight and simulated microgravity cause a significant reduction of key gene expression in early T-cell activation. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 308 (6), 480-488 (2015).
- Jong, J., Maki, G., Klingemann, H. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 8 (4), 652-658 (1994).
- Williams, B. A., et al. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell. Oncotarget. 8 (51), 89256-89268 (2017).
- Cryopreservation of mammalian cell lines video protocol. Abcam Available from: https://www.abcam.com/protocols/cryopreservation-of-mammalian-cell-lines-video-protocol (2022)
- Counting cells using a hemocytometer. Abcam Available from: https://www.abcam.com/protocols/counting-cells-using-a-haemocytometer (2022)
- Mylabathula, P. L., et al. Simulated microgravity disarms human NK-cells and inhibits anti-tumor cytotoxicity in vitro. Acta Astronautica. 174, 32-40 (2020).
- Li, Q., et al. Effects of simulated microgravity on primary human NK cells. Astrobiology. 13 (8), 703-714 (2013).
- Shao, D., et al. Mechanisms of the effect of simulated microgravity on the cytotoxicity of NK cells following the DNA methylation of NKG2D and the expression of DAP10. Microgravity Science and Technology. 33 (1), 6 (2021).
- Castro, S. L., Nelman-Gonzalez, M., Nickerson, C. A., Ott, C. M. Induction of attachment-independent biofilm formation and repression of hfq expression by low-fluid-shear culture of Staphylococcus aureus. Applied and Environmental Microbiology. 77 (18), 6368-6378 (2011).
- Phelan, M. A., Gianforcaro, A. L., Gerstenhaber, J. A., Lelkes, P. I. An air bubble-isolating rotating wall vessel bioreactor for improved spheroid/organoid formation. Tissue Engineering Part C: Methods. 25 (8), 479-488 (2019).

