A Ferret Model of Inflammation-sensitized Late Preterm Hypoxic-ischemic Brain Injury
Summary
The method describes inflammation-sensitized hypoxic-ischemic and hyperoxic brain injury in the P17 ferret to model the complex interaction between prolonged inflammation and oxidative brain injury experienced in a number of late preterm infants.
Abstract
There is an ongoing need for clinically relevant models of perinatal infection and hypoxia-ischemia (HI) in which to test therapeutic interventions for infants with the neurological sequela of prematurity. Ferrets are ideal candidates for modeling the preterm human brain, as they are born lissencephalic and develop gyrencephalic brains postnatally. At birth, ferret brain development is similar to a 13 week human fetus, with postnatal-day (P) 17 kits considered to be equivalent to an infant at 32–36 weeks' gestation. We describe an injury model in the P17 ferret, where lipopolysaccharide administration is followed by bilateral cerebral ischemia, hypoxia, and hyperoxia. This simulates the complex interaction of prolonged inflammation, ischemia, hypoxia, and oxidative stress experienced in a number of neonates who develop brain injury. Injured animals display a range of gross injury severity, with morphological changes in the brain including narrowing of multiple cortical gyri and associated sulci. Injured animals also show slowed reflex development, slower and more variable speed of locomotion in an automated catwalk, and decreased exploration in an open field. This model provides a platform in which to test putative therapies for infants with neonatal encephalopathy associated with inflammation and HI, study mechanisms of injury that affect cortical development, and investigate pathways that provide resilience in unaffected animals.
Introduction
There is an ongoing need for large animal models that reflect the pathophysiology of prematurity and perinatal hypoxia-ischemia in which therapeutic interventions for infants can be tested. In 2017, 9.93% of the 382,726 infants born in the United States were born preterm, and 84% of these infants were born between 32 and 36 weeks of gestation1. In premature infants, perinatal exposure to infection or inflammation is common, where maternal immune activation due to viral or bacterial pathogens can initiate preterm labor. Postnatally, preterm infants are at high risk of early or late onset sepsis2. Preterm infants also frequently experience periods of hypoxia, hypotension, and hyperoxia due to their immature cardiorespiratory system, elevated oxygen tension in the atmosphere relative to those experienced in utero, and iatrogenic exposures. Additionally, in preterm infants, antioxidant defenses are immature3 and pro-apoptotic factors are naturally upregulated4. Oxidative stress and cell death lead to activation of the immune system and neuroinflammation. These combined factors are thought to contribute to developmental and physiologic vulnerability of the brain, and result in or exacerbate the encephalopathy associated with poor developmental outcomes in preterm infants5,6,7.
Due to the physical and developmental similarities that the ferret brain shares with the human brain, the ferret is an attractive species in which to model brain injury8,9,10,11,12. Ferrets are also ideal candidates to model the preterm human brain, as they are born lissencephalic and develop gyrencephalic brains postnatally, which provides a window in which to expose the developing brain to insults that mimic those experienced by infants born preterm. At birth, ferret brain development is similar to a 13 week human fetus, with postnatal-day (P) 17 kits considered to be equivalent to an infant at 32–36 weeks of gestation13.
Our group has recently published a model of extremely preterm (<28 weeks' gestation) brain injury in the P10 ferret by combining inflammatory sensitization with Escherichia coli lipopolysaccharide (LPS) with subsequent exposure to hypoxia and hyperoxia12. In the following protocol, we now describe a late preterm model in the P17 ferret, where LPS sensitization is followed by bilateral cerebral ischemia, hypoxia, and hyperoxia. This results in more severe injury in a subset of animals, and more closely models the complex interaction of prolonged inflammation, ischemia, hypoxia, and oxidative stress experienced in a number of preterm infants who develop brain injury.
Protocol
Procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and as part of an approved protocol by the University of Washington Institutional Animal Care and Use Committee.
1. Preparation and LPS Administration
NOTE: Refer to Figure 1 for a timeline of the procedures.
- Prior to commencing the procedure, seal, sterilize and autoclave all surgical instruments and surgical drapes. Prepare pre-operative medications in sterile vials. Calculate the flow rate required to replace the air in the hypoxia/hyperoxia chamber with the experimental gas in 8–10 min.
- Prepare the lipopolysaccharide (LPS from E. coli 055:B5) in sterile saline to produce a concentration of 1 mg/mL. Remove P17 ferret kits from their jills. Weigh and number them. Randomize animals by litter and sex to the control or injured (or treatment) groups.
- Using a 300 µL insulin syringe, administer 3 mg/kg of LPS intraperitoneally to kits in the injury group, and an equivalent volume of sterile saline vehicle (3 µL/g) to control animals.
- Place animals in a chamber within a water bath at 37–40 °C in order to maintain a target rectal temperature of 36–37 °C throughout the surgical procedures.
2. Anesthesia
- During the procedure, continually monitor temperature, respiration rate, and heart rate of the animal.
- Administer buprenorphine (0.05 mg/kg) subcutaneously 30 min before the surgical procedure. Induce anesthesia in a mixture of 3% isoflurane balanced with 100% oxygen. Remove the kit from the induction chamber and place it supine on a draped surgical water blanket set to 37 °C. Transfer anesthesia to the nose cone and reduce isoflurane level to 2–3%.
3. Surgical Preparation
- Using small animal clippers remove all hair on the ventral neck region. Shave in a rectangular pattern with care to avoid nicking the skin or generating razor rash. Administer local anesthesia to the shaved area using intradermal lidocaine (4 mg/kg) and bupivacaine (2.5 mg/kg).
- Prepare the neck by alternating application of povidone-iodine and 70% ethanol scrub with sterile cotton swabs. Repeat the scrub such that povidone-iodine and 70% ethanol are each applied 3x in alternating fashion.
- Confirm depth of anesthesia via absence of toe-pinch reflex. Maintain level of isoflurane at the minimum percentage required for a surgical plane of anesthesia. Using sterile disposable cut-out drapes that expose the neck region, drape the animal.
4. Bilateral Carotid Artery Ligation
- With a single-use #11 scalpel blade, make a 1.5 cm midline incision in the center of the neck. Using fine hemostats and curved forceps, bluntly dissect down to the left carotid artery. Dissect the artery away from the associated neurovascular bundle.
- Using a pair of curved fine forceps, pass a looped 10 cm length of sterile 5-0 silk suture under the artery. Cut the suture in half. Ligate the artery by securely tying both lengths of suture, leaving at least 2 mm between the knots. Transect the left carotid artery between the sutures, taking care to leave the nerve intact.
- Repeat the dissection on the right side. Reversibly ligate the right carotid artery with a single sterile 1/8 inch umbilical tie. Close the wound with surgical skin clips.
- Allow the animal to recover in a temperature-controlled water bath for at least 30 min before hypoxia.
NOTE: If the arteries are not fully isolated from the rest of the neurovascular bundle, increased mortality may be seen before or during later hypoxia.
5. Sequential Hypoxia, Hyperoxia, and Hypoxia
- During hypoxia and hyperoxia, alter water bath temperature as necessary to maintain rectal temperature during hypoxia at 37 °C in the sentinel animal(s).
- Place animals in the injury group in an airtight chamber within a water bath. Continuously monitor oxygen concentration within the chamber, as well as rectal temperature in at least one sentinel animal. Flush the chamber with humidified 9% oxygen (91% nitrogen) then maintain a flow rate of 3–5 L/min, depending on chamber size. Once the concentration of oxygen in the chamber has reached 9%, continue for 30 min.
- After 30 min, switch gas supply to 80% humidified oxygen (20% nitrogen), and allow the chamber to reach the target concentration based on flow rate and chamber size. Continue for 30 min of hyperoxia. Open the chamber to allow it to more rapidly reach normoxia by equilibrating with room air.
- Seal the chamber, and flush with 9% humidified oxygen. Continuously monitor all animals visually, taking note of animals that display bradypnea. Once the concentration of oxygen in the chamber has reached 9%, continue for 30 min. If intra-hypoxic mortality (respiratory arrest) in any of the animals is seen before the end of the 30 min period, terminate hypoxia immediately.
6. Reversal of Right Carotid Artery Ligation
- Return animals to the surgical area, and induce anesthesia in a mixture of 3% isoflurane balanced with 100% oxygen. Transfer anesthesia to nose cone and reduce isoflurane level to 2–3%. Remove the surgical wound clips and re-prepare the wound area with povidone-iodine. Confirm depth of anesthesia via absence of toe-pinch reflex. Maintain the level of isoflurane at the minimum percentage required for a surgical plane of anesthesia. Using sterile disposable cut-out drapes that expose the neck region, drape the animal.
- Using curved forceps, identify and untie the umbilical tape from the right carotid artery. Close the wound with surgical skin clips.
7. Recovery and Temperature Management
- Return all kits to their jills for 60 min, for nursing and recovery. After 60 min, return injured animals to the water baths at 37–40 °C for 6 h, adjusting water temperature as needed in order to maintain rectal temperature at 36–37 °C. Return kits to their jills.
- Remove surgical clips 10–14 days after surgery (P27–P31).
8. Reflex Testing
- Perform all the reflex tests daily from P21–P28, and at least 3x per week from P28–P42, whilst remaining blinded to exposure (or treatment) group. Prior to reflex testing, place kits in a chamber with heat assistance (37 °C water bath, heat pad, etc.) for 1 h. For each test, complete all trials per kit before testing the next kit.
- Negative geotaxis (25°)
- Place a flat board (16 ½ in. x 12 in.) wrapped in an absorbent benchtop protector against an object so that the board forms a 25° angle with the table. Place a kit on the board prone and facing downhill, approximately 75% of the way up the board.
- Ensure the kit’s body is straight and that it has all four paws gripped against the board before releasing it. As soon as the kit is placed, start the time assessment.
- Record the time when the kit manages to rotate its body 90° relative to its starting position. Record the time at which the kit rotates its body 180° and takes a full step towards the top of the board. Perform 3 trials at the 25° incline before moving onto the next test.
- Negative geotaxis (45°)
- Perform 3 trials of the previously described negative geotaxis test again, this time with the board set to a 45° angle.
- Cliff aversion
- Place a padded platform around 1 foot below the ledge to minimize injury to kits if they fall.
- Place a kit facing, and perpendicular to, the edge of the lab bench. Ensure the kit's body is straight, with its front paws flush with the edge. Begin time assessment from the moment the kit is placed. Take care to differentiate between conscious movement away from the cliff and other spontaneous movements that do not involve coordinated walking.
- Record the time when the kit moves its body away from the edge (defined as the kit backing up, turning its body, or moving its front limbs away from the edge). Record the time the kit completes its first step in the opposite direction of the edge (defined as any direction or angle past a 90° rotation from its starting position facing the edge).
- Perform 3 cliff aversion trials per kit before moving onto the next test.
- Righting reflex
- Place a kit supine on the bench, holding it gently in that position before releasing it and simultaneously beginning the stopwatch. Record the time that the kit brings itself to rest with all four paws simultaneously flat against the bench in weight-bearing positions. Record the time when the kit takes a full step in any direction (defined as the placement of all four paws to achieve progress in a given direction with no spinning or dragging of the body).
- Perform 5 trials of the righting reflex test per animal.
- After every kit has completed the 5 righting reflex trials, return the litter to the jill.
9. Catwalk Testing
- On P42, remove kits from the jill. Place kits in plastic cages approximately 10 min before testing, so they can acclimate to the environment. Turn the lights off in the testing room to ensure ambient light does not affect catwalk function.
- Create a new experiment in the relevant software. Adjust experimental settings so that the maximum run duration does not exceed 10.00 s and the minimum run duration is not less than 1.50 s. Set the maximum speed variation so it does not exceed 60%. Set a minimum requirement of three compliant runs for each animal.
- Adjust the width of the walkway relative to the size of the animal so that it is able to freely locomote without touching the walls while remaining narrow enough to discourage turning. Add a new detection setting in the Detection settings profiles tab using auto detection. Use the same detection settings for all litters and animals at a given age.
- Thoroughly clean the walkway with a low-lint paper tissue and 70% ethanol before and after each animal. Clean the ferret's paws regularly to improve accuracy of detection and classification. Once the walkway and animal are prepared, begin trial acquisition.
- Pause acquisition to clean the catwalk if footprints accumulate on the glass, or if animals pass urine or feces. Stop acquisition once the catwalk software has recognized three compliant runs based on the pre-determined experiment settings.
10. Open Field Testing (P42)
- Use a non-porous acrylic box (55 cm x 55 cm x 40 cm high) painted matte-white. Position the camera so that it is centered directly above box and all four walls are captured. Clean the testing arena with 70% ethanol before first use and between animals.
- In the relevant software, select New From Template and Apply a Pre-defined Template. Continue the set-up procedure by sequentially selecting Subject type: Other; Arena template: Open field, square; Zone template: Center, border, corners; Features to track: Center-point.
- Open Arena Settings and grab the background image from the camera input, making sure that the tops of the arena walls are visible. Calibrate the dimensions of the arena using the scale tool.
- Adjust the pre-defined arena zones by resizing the outlines to fit the wall zones (NW, NE, SW, SE) and floor zones (left top, middle top, right top, left middle, center, right middle, left bottom, middle bottom, right bottom). Validate the set-up to confirm that no zones overlap.
- Open the Acquisition window and press Start Acquisition. Place the ferret at the center of the testing arena oriented in such a way that is consistent for every test subject. Allow the ferret to move freely throughout the arena for a period of 5 min. At the end of the testing period, press Stop Acquisition. Repeat the procedure with the next ferret.
NOTE: All experimenters in the room should position themselves to be unobservable by the ferret and remain quiet during the testing period.
11. Fixation-perfusion
- On P42, deeply anesthetize kits with 5% isoflurane. Administer a pentobarbital overdose (120–150 mg/kg i.p.). Ensure deep anesthesia by lack of response to toe pinch and loss of respiratory movements.
- Transfer the animal to a fume hood. Open the thorax and clamp the descending aorta using fine hemostats. Cut the right atrium. Using a perfusion pump, perfuse the left ventricle with 60 mL of sterile saline at a rate of 30 mL/min. Perfuse with 60 mL of formalin (10% formaldehyde) at a rate of 30 mL/min.
- Decapitate the carcass, and remove the brain from the skull using scissors, forceps, rongeurs, and a spatula. Take high-resolution photographs of the dorsal, ventral, and lateral aspects of each brain. Post-fix the brain in formalin for at least 48 h.
12. Ex Vivo Brain Measurement
- Remove the brain from formalin (step 11.3) and place onto a paper towel to absorb excess liquid.
- Using an electronic caliper, measure the height of the brain by placing the tips of the caliper at the dorsal and ventral aspects of the brain. Measure the length of the brain by placing the tips of the caliper at the olfactory bulb and the most posterior border of the occipital lobe. Measure the width of the brain by placing the tips of the caliper at the most lateral portions of the temporal lobes. Weigh the brain.
- Measure the longitudinal fissure (anterior and posterior to the cruciate sulcus), lateral sulci, suprasylvian sulci, coronal sulci, pseudosylvian sulci, ansinate sulci, cruciate sulci, presylvian sulci, lateral gyri, suprasylvian gyri, sigmoid gyri (anterior and posterior), coronal gyri, ectosylvian gyri (anterior and posterior), and orbital gyri. Measure all sulci from the beginning and end of the most distinct portion of the corresponding sulcus. Measure all gyri from the widest aspect of each corresponding gyrus.
- Measure the amount of cerebellum exposed by placing one tip of the caliper at the most posterior point of the longitudinal fissure and placing the other tip of the caliper at the most posterior part of the cerebellum.
NOTE: The ferret brain atlas, as found in Biology and Diseases of the Ferret14, was used to develop the ex vivo ferret brain measurements.
13. Gross Injury Scoring
- Using the photographs taken in step 11.3., apply the scoring criteria in steps 13.2–13.4 to assess gross brain injury (0–9 scale) whilst remaining blinded to exposure (or treatment) groups.
- Assess the longitudinal fissure. If it appears normal, assign a score of 0. If it is mildly widened (approximately 2x normal width), but the increase in width is incomplete along length of fissure, assign a score of 1. If it is moderately widened (approximately 2–3x normal), assign a score of 2. If it is markedly widened, with a visible gap >3x normal width along most of the length of fissure, apply a score of 3.
- Assess the lateral sulci. If they show normal definition, with separation of the lateral gyri and suprasylvian gyri, assign a score of 0. If mild unilateral or bilateral reduced definition of sulcus is seen, particularly in the caudal portion, with minimal narrowing of frontal and temporal lobes relative to the occipital lobes, assign a score of 1.
- If moderately reduced definition of the sulci is seen, with depression of the suprasylvian gyri, narrowing of the coronal and ectosylvian gyri, and mild narrowing of frontal and temporal lobes relative to occipital lobes, assign a score of 2. If unilateral cystic degeneration is seen, with minimal change of the contralateral hemisphere, assign a score of 3. If poor definition of the lateral sulci is present, with bilateral cystic or severe degeneration of the occipital and temporal lobes, assign a score of 4.
- Assess the visible portion of the cerebellum. If it appears normal, with (<75% of the vermis and <66% of the hemispheres visible, assign a score of 0. If 75–90% of the vermis and ≥66% of the hemispheres are visible, assign a score of 1. If most of the cerebellum is visible, showing all of the vermis and ≥66% of hemispheres, assign a score of 2.
14. Data Analysis
- For reflex testing data, assign failures a score of 61 s to allow them to be compared to successes at the end of time (60 s), but with a worse ranking in the statistical analysis. Calculate an area under the curve for each animal over time in each of the reflex tests.
- Adjust catwalk data that involves paw size of pressure by the weight of the animal.
- Analyze data using non-parametric statistical methods, describing data using the median and interquartile range (IQR).
Representative Results
Of 34 (n = 18 males, n = 16 females) animals from six litters exposed to the insult, eight animals (24%; n = 4 males, n = 4 females) in the injured group died during the second hypoxia period (n = 5), during temperature management (n = 2), or overnight after the insult (n = 1). In the injured group, nine of 26 survivors (35%) had visible gross injury. Five animals (n = 5 males) had moderate injury, and four animals (n = 2 males, n = 2 females) had severe injury, defined as gross pathology scores of 2–5 and 6–9, respectively (Figure 2A). Animals exposed to the insult therefore have a 50% risk of death or significant gross injury. With increasing injury, narrowing of gyri in the temporal and/or occipital lobes is seen, with associated sulcal shortening, widening of the longitudinal fissure, and large areas of areas of cystic tissue loss in the most severely-injured animals (Figure 2B). In surviving injured animals (n = 26; n = 14 males, n = 12 females), significantly greater exposure of the cerebellum is seen (Figure 3A), as well as shortening of the longitudinal fissure (Figure 3D). There is also significant narrowing of the coronal and anterior ectosylvian gyri (Figure 3B,E), as well as shortening of the lateral and suprasylvian sulci (Figure 3C,F). Median (IQR) brain weight was 8.1 g (7.9–9.7g, n = 6) in control animals, and 7.0 g (6.5–7.7 g) in injured animals (n = 26, p = 0.005). In control animals, median (IQR) brain length was 28.9mm (27.8–29.6 mm, n = 6) compared to 27.5 mm (25.5–38.0 mm, n = 26) in injured animals (p = 0.007). Similar patterns are seen across the brain, with median width and height 5–7% smaller in injured animals. Anatomical structures on both the left and right side are affected in a similar manner, with no difference between hemispheres. See Figure 1B for depictions of the anatomical locations. Over the reflex testing period (P21–P39), injured animals display slower time to rotate in the negative geotaxis task (Figure 4A), slower time to rotate away from the edge in the cliff aversion task (Figure 4B), and slower time to right (Figure 4C). In the catwalk, injured animals have a similar average speed to controls (Figure 5A), but display a significantly greater degree of speed variation during each run (Figure 5B). The weight-adjusted distance between fore paws and hind paws (print position) is significantly greater in injured animals (Figure 5C), with less pressure exerted per unit paw area through the fore paws (Figure 5D). In the open field, injured animals cover less total distance (Figure 6A), and stop more frequently (Figure 6B). They spend significantly more time in the center of the field, and less time in the corners (Figure 6C,D). Representative heat maps of control and injured animals are shown in Figure 7A,B.
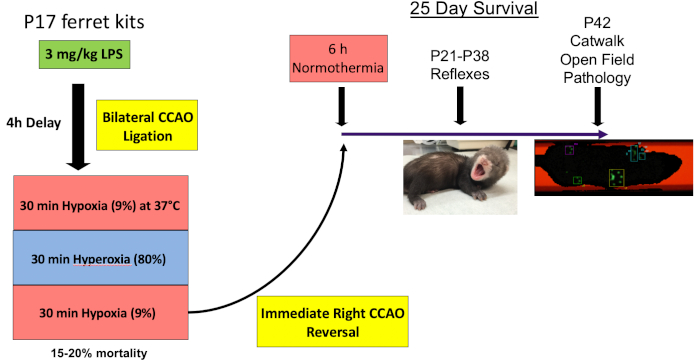
Figure 1: Timeline. On P17, animals are administered 3 mg/kg LPS before undergoing bilateral carotid artery ligation and 30 min each (not including time for the chamber to equilibrate) of hypoxia (9% oxygen), hyperoxia (80% oxygen) and hypoxia (9%). The right carotid artery ligation is then reversed. Animals are exposed to 6 h of normothermia to ensure they do not become spontaneously hypothermic in the nest in the period after injury. Reflex testing is then performed daily from P21–P28, and three times per week from P28–P42. On P42, animals are tested in the catwalk and open field before sacrifice. Please click here to view a larger version of this figure.
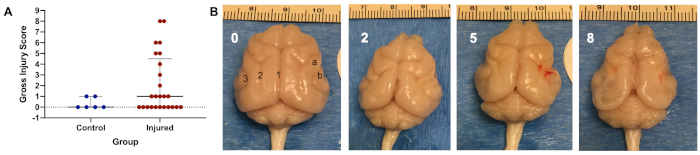
Figure 2: Representative injury distribution and depiction. (A) Gross injury scoring from 26 survivors (n = 14 males, n = 12 females) in the injured group, compared to six litter mate controls. Five animals (n = 5 males) had moderate injury, and four animals (n = 2 males, n = 2 females) had severe injury, defined as gross pathology scores of 2–5 and 6–9, respectively. Graph shows median with interquartile range. (B) Control brain (left panel, score 0), with brains depicting increasing gross injury scores of 2, 5, and 8 out of a total possible score of 9, from left to right. The control brain shows anatomical structures particularly susceptible to injury; 1 = longitudinal fissure, 2 = lateral sulcus, 3 = suprasylvian sulcus, a = coronal gyrus, b = anterior ectosylvian gyrus. Please click here to view a larger version of this figure.
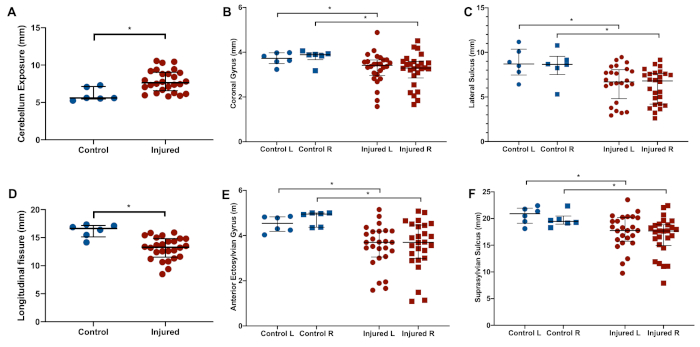
Figure 3: Representative brain measurements. Compared to controls (n = 6), injured animals (n = 26) display significantly increased exposure of the cerebellum (A), shortening of the longitudinal fissure (D), narrowing of the coronal (B) and anterior ectosylvian (E) gyri, and shortening of the lateral (C) and suprasylvian (F) sulci. Graphs show median with interquartile range. *denotes p < 0.05 (Wilcoxon-Mann-Whitney U-test). Please click here to view a larger version of this figure.
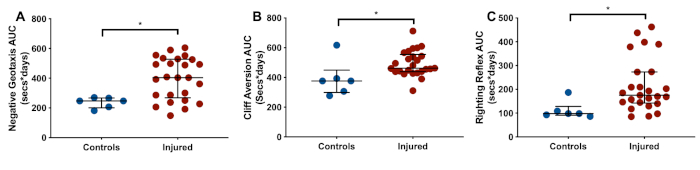
Figure 4: Representative reflex development. Compared to controls (n = 6), injured animals (n = 26) display slower development (area under the curve, AUC) of negative geotaxis (A), cliff aversion (B), and righting reflex (C). Graphs show median with interquartile range. *denotes p < 0.05 (Wilcoxon-Mann-Whitney U-test). Please click here to view a larger version of this figure.
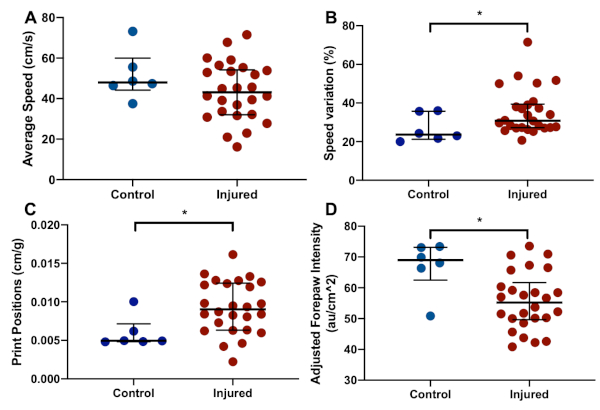
Figure 5: Representative catwalk results. Compared to controls (n = 6), injured animals (n = 26) walk at a similar average pace (A), but with a greater variability in speed during walking (B). Injured animals also display a longer average print position (C), with less pressure applied per unit area (D). Graphs show median with interquartile range. * denotes p < 0.05 (Wilcoxon-Mann-Whitney U-test). Please click here to view a larger version of this figure.
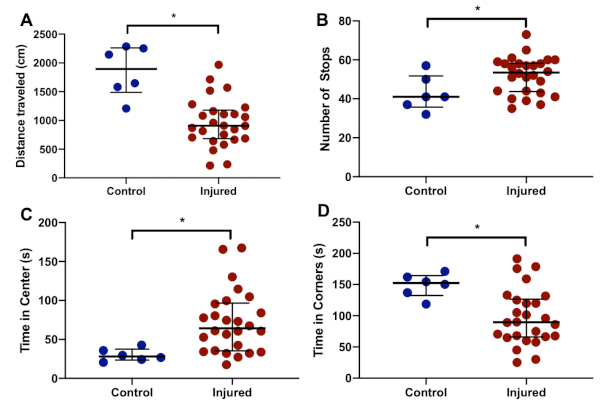
Figure 6: Representative open field behavior. Compared to controls (n = 6), injured animals (n = 26) cover a smaller total distance (A), as well as stopping more frequently (B). Injured animals also spend more time at the center (C) than in the corners (D). Graphs show median with interquartile range. *denotes p < 0.05 (Wilcoxon-Mann-Whitney U-test). Please click here to view a larger version of this figure.
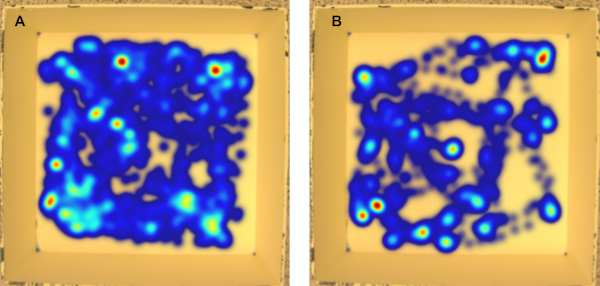
Figure 7: Representative open field heat maps. (A) control female, (B) injured female. Injured animals cover a significantly smaller distance within the open field. Please click here to view a larger version of this figure.
Discussion
Due to the physical and developmental similarities shared between the ferret brain and human brain, the ferret is increasingly being used to model both adult and developmental brain injury.8,9,10,11,12. However, research to date suggests that the ferret brain is both resistant to initial injury as well as highly-plastic, with behavioral deficits diminishing over time even in the setting of visible pathological injury10,12. Here, we describe the first model of inflammation-sensitized hypoxic-ischemic (HI) brain injury in the late preterm-equivalent ferret, which results in significant bilateral injury and sustained behavioral deficits in survivors. As with any preclinical model, the goal was not to accurately reproduce the exposures encountered by preterm infants clinically, but to provide a confluence of the mechanistic factors thought to be involved in premature brain injury. These include inflammation, hypoxia, and oxidative stress7.
One critical aspect of LPS administration in our ferret models is a single high dose given around 4 h before hypoxia. LPS exposure in near-term equivalent rodents results in a circulating inflammatory cytokine peak around 4 h after exposure, which corresponds with sensitization of the brain to hypoxia-ischemia, and a significant increase in brain injury15,16,17. A similar time course of inflammatory cytokine release (peak TNF-α and IL-6 release 2–4 h after LPS exposure) is seen in isolated ferret peripheral blood mononuclear cells18. Assuming a single surgical set-up, administering LPS 30–60 min before the start of surgery allows for adequate time to perform 12–15 bilateral carotid artery ligations and initiate the first hypoxia exposure 4 h after LPS administration. During model development, an LPS dose of 5 mg/kg was initially used, as described in our P10 injury model12. However, this LPS dose was associated with significant intra-hypoxic mortality and pulmonary edema on necropsy. Both mortality and pulmonary edema were reduced by decreasing the LPS dose to 3 mg/kg.
During hypoxia exposure, a number of factors appear to be critical to ensuring significant gross injury whilst also preventing high levels of mortality. Due to laboratory ferrets being outbred, there is an inherent variability in hypoxia tolerance across litters. In our experience, cross-fostering animals or combining animals from different litters in the same hypoxia chamber predominantly results in the earlier death of larger animals or animals from the most susceptible litter. If more susceptible animals die before the target 30 min hypoxia exposure and hypoxia is stopped early, smaller animals from less susceptible litters will receive suboptimal hypoxia exposure, and are unlikely to sustain significant injury. As a result, each litter of animals should be exposed to hypoxia within their own separate chamber. The second hypoxia period was added as part of an iterative model development process that we have previously described12. A single hypoxia period resulted either in death or survival with no significant injury, regardless of length.
As ferrets are able to tolerate long periods of acute hypoxia or bilateral carotid artery ligation without showing significant brain injury, our current hypothesis is that the period of hyperoxia results in elevated metabolism and vasodilatation that facilitates brain ischemia during the second hypoxia period. To minimize variability in the model, we used pre-ordered sex-balanced litters of 8 ferrets that arrived in our facility on P15. In each litter, 6–7 animals underwent surgery followed by hypoxia within a single chamber.
After hypoxia and reversal of the right carotid artery ligation, animals should be returned to their jills for a period of time to feed due to a risk of dehydration and hypoglycemia from the prolonged injury protocol. If significant mortality is experienced during the temperature management period, animals may need additional fluid resuscitation (subcutaneous saline and/or hand feeding with formula and water) before being placed in the water baths for 6 h. The temperature management period is, however, a critical determinant of long-term injury, as animals may otherwise experience neuroprotection from relative hypothermia in the nest. This risk of hypothermia is at least partly due to the low temperature of housing conditions required for the ferret (60–70 °F).
The behavioral tests described were largely developed within the laboratory, with some basis in reflex tests previously described in the developing ferret19, with catwalk and open field tests adapted from adult rodents to be used in juvenile ferrets. Other groups have also described open field, maze, and gait testing in adult ferrets after traumatic brain injury10, as well as the effect of in utero inflammation on social interaction in adult ferrets20. Though a long fasting period is not recommended in ferrets due to their short gut transit time, placing them in an animal carrier for 30–60 min before any of the tests is beneficial in order to allow them to pass urine and feces before the tests. As the ferret is by nature an inquisitive animal, it often behaves in an opposite manner to rodents in these behavioral tests. This is particularly evident in the catwalk, where lights and sounds, particularly recordings of another ferret vocalizing ("dooking"), can be used to motivate the ferret to walk forwards.
The current protocol does have some limitations. As it was developed iteratively using previously developed methods in the P10 ferret12, we do not currently know the relative contributions of LPS, hypoxia, hyperoxia, and reperfusion to the final degree of injury seen. However, it is worth noting that development of the method described here included using the original Vannucci model (unilateral carotid artery ligation followed by a single period of hypoxia) in the ferret21, which did not result in any significant injury. Therefore, interactions between the multiple parts of the injury protocol is likely to be necessary for sustained injury. Despite this, there remains a distinct variability in gross injury in surviving animals, which is another potential limitation. Though animals without significant gross injury may have injury that is detectable using MRI or histopathology12, future work on the model will include iterations to try and increase the number of animals that sustain significant injury, for instance by using permanent bilateral carotid artery ligation. Finally, in order for this model to be maximally useful to test putative neuroprotective therapies for developmental brain injury, it should be validated by assessing the efficacy of neuroprotective agents that are either established for the treatment of HI brain injury in human neonates, or have been successful in a range of other animal models of neonatal brain injury. Future studies will therefore assess the efficacy of therapeutic hypothermia and erythropoietin in this model, including sex-based therapeutic responses and ex vivo MRI12.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
Development of the model was funded Bill and Melinda Gates Foundation, as well as by NIH grant 5R21NS093154-02 (NICHD).
Materials
| 80% Oxygen | Praxair | ||
| 9% Oxygen | Praxair | ||
| Absorbent benchtop protector | Kimtech | 7546 | |
| Automated catwalk | Noldus | ||
| Betadine surgical scrub | |||
| Bupivacaine | Patterson Veterinary | 07-888-9382 | |
| Buprenorphine | |||
| Calipers | SRA Measurement Products | ME-CAL-FP-200 | 200mm range, .01 mm resolution |
| Cotton Gauze Sponge | Fisher Scientific | 22028556 | |
| Curved fine hemostat | Roboz | RS-7101 | |
| Curved forceps | World Precision Instruments | 501215 | |
| Curved suture-tying hemostat | Roboz | RS-7111 | |
| Ethovision tracking software | Noldus | ||
| Eye Lubricant | Rugby | NDC 0536-1970-72 | |
| Ferrets (Mustela putorius furo) | Marshall Biosciences | Outbred (no specific strain) | |
| Formalin | Fisher Scientific | SF100-4 | 10% (Phosphate Buffer/Certified) |
| Hair Clippers | Conair | GMT175N | |
| Insulin Syringes | BD | 329461 | 0.3 cc 3 mm 31G |
| Isoflurane | Piramal | 66794-017-25 | |
| Lidocaine | Patterson Veterinary | 07-808-8202 | |
| LPS | List Biological | LPS Ultrapure #423 | |
| Oxygen sensor | BW Gas Alert | GAXT-X-DL-2 | |
| Pentobarbital | |||
| Plastic chamber | Tellfresh | 1960 | 10L; 373x270x135mm |
| Saline Solution, 0.9% | Hospira | RL-4492 | |
| Scalpel blade | Integra Miltex | 297 | |
| Scalpel handle | World Precision Instruments | 500236 | #3, 13cm |
| Sterile suture | Fine Science Tools | 18020-50 | Braided Silk, 5/0 |
| Surgical clip applicator | Fine Science Tools | 12020-09 | |
| Surgical clip remover | Fine Science Tools | 12023-00 | |
| Surgical drapes | Medline Unidrape | VET3000 | |
| Surgical gloves | Ansell Perry Inc | 5785004 | |
| Surigical clips | Fine Science Tools | 12022-09 | |
| Thermometer (rectal) | YSI | Precision 4000A | |
| Thermometer (water) | Fisher Scientific | 14-648-26 | |
| Umbilical tape | Grafco | 3031 | Sterile |
| Water bath | Thermo Scientific | TSCOL19 | 19L |
Referencias
- Martin, J. A., Hamilton, B. E., Osterman, M. J. K., Driscoll, A. K., Drake, P. Births: Final Data for 2017. National Vital Statistics Report. 67 (8), 1-49 (2018).
- Vanhaesebrouck, P., et al. The EPIBEL study: outcomes to discharge from hospital for extremely preterm infants in Belgium. Pediatrics. 114 (3), 663-675 (2004).
- Raju, T. N., et al. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health. Journal of Pediatrics. , (2016).
- Raju, T. N. K., Buist, A. S., Blaisdell, C. J., Moxey-Mims, M., Saigal, S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatrica. 106 (9), 1409-1437 (2017).
- Bennet, L., et al. Chronic inflammation and impaired development of the preterm brain. Journal of Reproductive Immunology. 125, 45-55 (2018).
- Reich, B., Hoeber, D., Bendix, I., Felderhoff-Mueser, U. Hyperoxia and the Immature Brain. Developmental Neuroscience. 38 (5), 311-330 (2016).
- Galinsky, R., et al. Complex interactions between hypoxia-ischemia and inflammation in preterm brain injury. Developmental Medicine & Child Neurology. 60 (2), 126-133 (2018).
- Empie, K., Rangarajan, V., Juul, S. E. Is the ferret a suitable species for studying perinatal brain injury. International Journal of Developlemental Neuroscience. 45, 2-10 (2015).
- Snyder, J. M., et al. Ontogeny of white matter, toll-like receptor expression, and motor skills in the neonatal ferret. International Journal of Developlemental Neuroscience. , (2018).
- Schwerin, S. C., et al. Progression of histopathological and behavioral abnormalities following mild traumatic brain injury in the male ferret. Journal of Neuroscience Research. 96 (4), 556-572 (2018).
- Rafaels, K. A., et al. Brain injury risk from primary blast. Journal of Trauma and Acute Care Surgery. 73 (4), 895-901 (2012).
- Wood, T., et al. A Ferret Model of Encephalopathy of Prematurity. Developlemental Neuroscience. , (2019).
- Barnette, A. R., et al. Characterization of Brain Development in the Ferret via Magnetic Resonance Imaging. Pediatric Research. 66 (1), 80-84 (2009).
- Kroenke, C. D., Mills, B. D., Olavarria, J. F., Neil, J. J. . Biology and Diseases of the Ferret. , (2014).
- Eklind, S., et al. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. European Journal of Neuroscience. 13 (6), 1101-1106 (2001).
- Falck, M., et al. Neonatal Systemic Inflammation Induces Inflammatory Reactions and Brain Apoptosis in a Pathogen-Specific Manner. Neonatology. 113 (3), 212-220 (2018).
- Osredkar, D., et al. Hypothermia Does Not Reverse Cellular Responses Caused by Lipopolysaccharide in Neonatal Hypoxic-Ischaemic Brain Injury. Developmental Neuroscience. 37 (4-5), 390-397 (2015).
- Nakata, M., Itou, T., Sakai, T. Quantitative analysis of inflammatory cytokines expression in peripheral blood mononuclear cells of the ferret (Mustela putorius furo) using real-time PCR. Veterinary Immunology and Immunopathology. 130 (1-2), 88-91 (2009).
- Christensson, M., Garwicz, M. Time course of postnatal motor development in ferrets: ontogenetic and comparative perspectives. Behavioral Brain Research. 158 (2), 231-242 (2005).
- Li, Y., Dugyala, S. R., Ptacek, T. S., Gilmore, J. H., Frohlich, F. Maternal Immune Activation Alters Adult Behavior, Gut Microbiome and Juvenile Brain Oscillations in Ferrets. eNeuro. 5 (5), (2018).
- Rice, J. E., Vannucci, R. C., Brierley, J. B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Annals of Neurolology. 9 (2), 131-141 (1981).

