Surface Engineering of Pancreatic Islets with a Heparinized StarPEG Nanocoating
Summary
This protocol aims to achieve surface engineering of pancreatic islets using a heparin-incorporated starPEG nanocoating via pseudo-bioorthogonal chemistry between the N-hydroxysuccinimide groups of the nanocoating and the amine groups of islet cell membrane.
Abstract
Cell surface engineering can protect implanted cells from host immune attack. It can also reshape cellular landscape to improve graft function and survival post-transplantation. This protocol aims to achieve surface engineering of pancreatic islets using an ultrathin heparin-incorporated starPEG (Hep-PEG) nanocoating. To generate the Hep-PEG nanocoating for pancreatic islet surface engineering, heparin succinimidyl succinate (Heparin-NHS) was first synthesized by modification of its carboxylate groups using N-(3-dimethylamino propyl)-N’-ethyl carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS). The Hep-PEG mixture was then formed by crosslinking of the amino end-functionalized eight-armed starPEG (starPEG-(NH2)8) and Heparin-NHS. For islet surface coating, mouse islets were isolated via collagenase digestion and gradient purification using Histopaque. Isolated islets were then treated with ice cold Hep-PEG solution for 10 min to allow covalent binding between NHS and the amine groups of islet cell membrane. Nanocoating with the Hep-PEG incurs minimal alteration to islet size and volume and heparinization of the islets with Hep-PEG may also reduce instant blood-mediated inflammatory reaction during islet transplantation. This “easy-to-adopt” approach is mild enough for surface engineering of living cells without compromising cell viability. Considering that heparin has shown binding affinity to multiple cytokines, the Hep-PEG nanocoating also provides an open platform that enables incorporation of unlimited functional biological mediators and multi-layered surfaces for living cell surface bioengineering.
Introduction
The therapeutic efficacy of cell-based therapies is limited by low cell retention and poor survival1,2. In order to improve the outcome of cell therapies, cell surface engineering via enzymatic manipulation, peptide conjugation, bioorthogonal chemistry and physical encapsulation with biomaterials has been exploited3,4,5,6,7,8,9,10. The current protocol aims to achieve surface engineering of living cells using an "easy-to-adopt" method by applying an ultrathin heparin-incorporated starPEG (heparin-PEG) nanocoating to the cell surface. Surface engineering of pancreatic islets was presented here as an example due to the heterogeneous nature of islets of Langerhans and the disparaging outcomes of current clinical islet transplantation.
Indeed, clinical islet transplantation is currently performed by direct injection of isolated islets into the hepatic portal vein and this procedure is only available for selective patients because of the scarcity of donor materials and low therapeutic efficacy11. Conventionally, alginate has been the most commonly used biomaterial for islet encapsulation and surface modification, although it is less than ideal due to the chemical instability of alginate and inflammatory-related fibrosis12,13. Furthermore, compared to the natural size of islets that ranges between 100 to 200 µm, the alginate-islet microcapsules are larger, ranging between 400 and 800 µm, which exceed the physiological diffusing distance of oxygen. Conformal islet encapsulation, i.e., encapsulating islets without significant alteration of islet volume, was then developed. Thus, deposition of nanomembranes composed of PEG, tetrafluoroethylene, silicon membrane or multi-layered nanocoating (also known as the "layer-by-layer" [LBL] technique) has been reported, resulting in improved in vitro islet survival14,15,16,17,18, although the LBL approach often requires extensive islets handing period for deposition of multiple layers, which may compromise islet viability. Moreover, instability of nanomembranes that relies on electrostatic or covalent interactions between biomembrane layers or hydrophobic interactions between nanomembranes and the islet surface also raises concerns9,14,15,16,22,23,24,25,26.
Another limiting factor that encumbers the therapeutic outcome of intraportal islet transplantation is the instant blood-mediated inflammatory reaction (IBMIR) caused by direct contact of implanted islets with blood, resulting in platelet aggregation, coagulation and adverse immune effect or undesired cellular activation9. To address these problems, an ultra-thin nanocoating composed of star-shaped polyethylene glycol (starPEG) was prepared for its established biocompatibility and versatility as islet housing material. Heparin, a highly sulphated glycosaminoglycan, was also incorporated in the starPEG nanocoating for its anti-inflammatory, anti-coagulant properties and ability to facilitate vascularization by recruiting pro-angiogenic growth factors22,23.
Protocol
1. Fabrication of Heparin-incorporated Starpeg Nanocoating
- Synthesis of heparin succinimidyl succinate (Heparin-NHS)
- Weigh out 0.69 g of heparin and dissolve in 2.0 mL of ice-cold deionized water.
- Weigh out 0.23 g of NHS and dissolve in 0.5 mL of ice-cold deionized water in a round-bottom flask.
- Weigh out 0.77 g of EDC and dissolve in 0.5 mL of ice-cold deionized water in a round-bottom flask.
- Mix the NHS and EDC solutions in a round-bottom flask. Leave the mixed solution on the bench at room temperature for 30 min at room temperature.
- Centrifuge the EDC NHS mixture at 5,031 x g for 10 min to remove excess EDC and NHS.
- Add 30 mL of cold ethanol to the mixture solution and centrifuge at 5,031 x g for 10 min.
- Repeat twice.
- Preparation of starPEG-heparin nanocoating
- Add 1.0 mL of 10% (w/v) starPEG-(NH2)8 to a round-bottom flask.
- Add 0.67 mL of 10% (w/v) heparin-NHS to the same round-bottom flask.
- Place the mixed solution of starPEG-(NH2)8 and heparin-NHS in an incubator at 25 °C for 20 min to obtain a clear solution with viscosity.
NOTE: For experiments in which fluorescently labeled heparin (FAM-heparin) was required, the fabrication procedure is the same except that the star-PEG-(NH2)8 was dissolved in deionized water supplemented with 5(6)-carboxyfluorescein N-succinimidyl ester 0.1% (molar ratio) of starPEG-(NH2)8. )
- Characterization of heparin-PEG nanocoating by Fourier Transform Infrared Spectroscopy (FT-IR)
- Before starting, rinse the agate mortar, pestle and pelleting device (small disks with diameter of 13 mm) with acetone and deionized water. Dry the glassware in an oven before use.
- Mix approximately 0.1–1% heparin-PEG sample with 200–250 mg of fine KBr powder.
- Place the heparin-PEG and KBr mixture in the agate mortar and finely pulverize into small pellets with diameters of 2 µm.
- Transfer the mixture into a pelleting device. Apply high compression force (8 T/cm2) in a vacuum for 1–2 min to form transparent pellets.
- Gently pull the sample pellets away from the pelleting device. Be careful not to pull too hard so not to incur cracks.
- Transfer the sample pellets very carefully to a Fourier Transform Infrared Spectroscope to determine sample chemical structure.
- Examination of the internal porous structures of heparin-PEG nanocoating by scanned electron microscopy (SEM)
- For the following procedures, prepare standard 48-well cell culture plate and pipettes.
- Pipette the heparin-PEG copolymer solution into the wells of a 48-well culture plate.
- Freeze the plate at -80 °C for 24 h.
- Lypophilize the samples at -50 °C in vacuum environment (0.1 Pa) for 24 h.
- Spread samples across the sticky surface of an aluminum stub.
- Coat the samples with gold and observe under the scanned electron microscope to observe internal porous structures.
- Examination of the heparin-PEG nanocoating surface by atomic force microscopy (AFM)
- Clean silica glass slides with piranha solution (70% H2SO4, 30% H2O2) at 80 °C for 40 min.
- Sonicate the slides in methanol for 10 min and then in toluene for 10 min.
- Dry the slides by vacuum drying.
- Wash the slides with plenty 3-aminopropyl-triethoxysilane solution (2% in toluene), shake gently.
- Sonicate the slides in methanol for 10 min then in toluene for 10 min.
- Place 100 µL of 3% heparin-PEG solution across the surface of the slides using a standard pipette.
- Examine the surface of heparin-PEG under an atomic force microscope (a resonant frequency of 50–80 kHz, force constant of 0.350 N m 21, tip radius of 600 nm).
2. Mouse Islet Surface Engineering with Heparin-PEG Nanocoating
- Mouse islet surface coating with heparin-PEG nanocoating
- Extract mouse islets by collagenase digestion and histopaque gradient purification as previously reported in JoVE19.
- Handpick 200 islets under a dissection microscope to a 1.5 mL tube.
- Add 500 µL of PBS to the islets and centrifuge at 503 x g for 1 min.
- Take off the supernatant and add 250 µL of the heparin-PEG solution and keep the mixture on ice for 10 min. Pipette up and down to allow the islets to mix well with the heparin-PEG solution.
- Centrifuge at 503 x g for 1 min.
- Remove the supernatant and add 500 µL of PBS. Pipette up and down to mix well.
- Centrifuge at 503 x g for 1 min and remove the supernatant and keep the islets for further use. For examination of mouse islets coated with the heparin-PEG, FAM-heparin-PEG was used for islet coating following these steps.
- Examination of mouse islets coated with the FAM-heparin-PEG nanocoating
- Add 1–2 mL of RPMI 1640 supplemented with 10% fetal bovine serum to the coated islets and transfer these islets into a non-charged sterile bacterial culture dish.
- Observe the morphology and fluorescence signals of FAM-heparin-PEG nanocoating-coated islets under an inverted fluorescence microscope.
3. Function of Heparin-PEG Coated Mouse Islets Compared to Non-coated Islets
- Viability of heparin-PEG coated mouse islets
- Handpick 20 of the heparin-PEG coated mouse islets and place them into one well of a 96-well cell culture plate.
- Fill a total of 10 wells with 20 heparin-PEG coated mouse islets for each well.
- Handpick 20 of noncoated control islets and place them into one well of the same 96-well cell culture plate.
- Fill a total of 10 wells with 20 noncoated control islets for each well.
- Put 200 µL of RPMI 1640 supplemented with 10% fetal bovine serum into each well of the 96-well plate that contains islets and maintain in culture for 14 days.
- Replace islet culture media every 2 days.
- Treat the islets with a live/dead staining kit at the end of 14 days in culture and observed under an inverted fluorescence microscope.
- Count PI+ (red) cells within each islet under an inverted fluorescence microscope.
- Revascularization of the heparin-PEG coated mouse islets in vitro
- Pre-cool all experimental plastics including culture plate and pipette tips at least 12 h before use.
- Place a 24-well place on a cooling pad. Add 250 µL of ice cold growth factor-reduced Matrigel into each well of a 24-well cell culture plate. Place the coated 24-well plate at 4 °C for at least 30 min.
- While the matrix solution is setting in the fridge, trypsinize the mouse pancreatic islet endothelial Mile Sven 1 (MS1) cells.
- Remove all culture media from the tissue culture flask. Rinse the flask with 5 mL of PBS.
- Remove the PBS and add 3 mL of trypsin-EDTA. Incubate at 37 °C for 3 min in a standard incubator.
- Lightly tap the bottom of the flask to detach the cells and add 5 mL of serum-supplemented media.
- Collect the cells into a 15 mL centrifuge tube and centrifuge at 1,000 x g for 5 min.
- Take off the supernatant and add 5 mL of PBS. Pipette up and down to mix well.
- Centrifuge at 1,000 x g for 5 min and take off the supernatant.
- Add serum-supplemented DMEM media into the tube and seed 50,000 cells into each well of the matrix-solution-coated 24-well plate.
- Add 5 heparin-PEG coated islets or noncoated control islets into each well.
- Add serum-supplemented DMEM media into each well to a total of 500 µL per well.
- Observe tube formation after 4h and 24 h incubation under a light microscope.
- Glucose-stimulated insulin secretion of heparin-PEG coated islets
NOTE: To assess whether nanocoating would affect the function of mouse islets, glucose-stimulated insulin secretion of heparin-PEG coated and noncoated islets was assessed.- Handpick 30 heparin-PEG coated islets or noncoated control islets into one 1.5 mL tube. Prepare a total of 10 tubes for coated islets and noncoated islets each.
- Add 1 mL of physiological salt solution supplemented with 2 mmol/L glucose20 and incubate at 37 °C for 2 h.
NOTE: For the recipe of the physiological salt solution, please refer to Table 1. Keep the cap of tubes loose during incubation in the incubator. - Remove as much solution as possible.
- Stimulate islets by adding 600 µL of physiological salt solution supplemented with 20 mmol/L glucose at 37 °C for 30 min.
- Collect 200 µL of supernatant from each tube for insulin ELISA quantification using a commercial insulin ELISA kit.
Representative Results
The heparin-PEG nanocoating was synthesized by conjugation of starPEG-(NH2)8 and heparin using EDC and NHS as coupling agents (Figure 1). Chemical structure of the heparin-PEG nanocoating was examined by FT-IR and as shown in Figure 2, characteristic peaks of heparin could be observed at 3,300–3,600 cm-1, corresponding to the hydroxyl groups of heparin (Figure 2 red). The decrease in amplitude of the peak at 3,300–3,600 cm-1 (Figure 2 blue) represents conjugation between the starPEG-(NH2)8 amide groups and heparin carbonyl group. Amplitude of peak 1,650 cm-1 corresponds to the amide carbonyl stretching vibration was also reduced, indicating sufficient reaction between the carboxylate groups of heparin with succinimidyl succinate and amine of starPEG-(NH2)8. Surface structure of the heparin-PEG nanocoating was examined by atomic force microscopy and it is shown from Lou et al., the nanocoating was approximately 30 nm in height and 2 µm in width with small porous features (dark spots) ranging between 100 to 200 nm in diameter10. Data obtained from scanned electronic microscopy also confirm the highly interconnected porous structure of the heparin-PEG (Figure 3), suggesting that it could be suitable for cell survival during in vivo delivery.
Surface coating of isolated mouse islets was examined and as presented in Figure 4, thin layer of nanocoating, shown by green fluorescence was evenly deposited across the surface of coated islets without causing evident changes on islet volume/size. During coating, it is recommended to keep the islets on ice to maintain their viability. Similarly, the coating period, 10 min in this case, was also optimized to allow maintenance of islets viability. It is worth noting that for better observation of the nanocoating, electron microscopy that examines the cross-sections of coated islets as previously reported9,16 would be more appropriate, although the data would be generated from fixed and embedded islets instead of living islets in culture.
Regarding survival and function of coated islets, islet revascularization and functions in have been assessed in vitro. Considering the beneficial properties of heparin, heparin functionalization onto the islet surface could facilitate islet revascularization in culture and consequently its survival. We have observed that the heparin-PEG coated mouse islets exhibited robust islet viability in culture (Figure 5). Significantly more advanced vascular formation was also evident from islet endothelial cells (MS1) that were co-cultured with heparin-PEG coated islets, indicated by elongated microvessel-like structures and network-like vascular structures (Figure 6).
The nanocoating process incurred no effect glucose-stimulated insulin secretory ability of the heparin-PEG coated islets. Low level of insulin secretion was observed in all treatment groups when islets were perfused with physiological salt solution supplemented with sub-stimulatory level of glucose (2 mmol/L; Figure 7). When islets were stimulated with a supra-physiological level of glucose (20 mmol/L glucose), increase in insulin secretion was observed in all treatment groups.
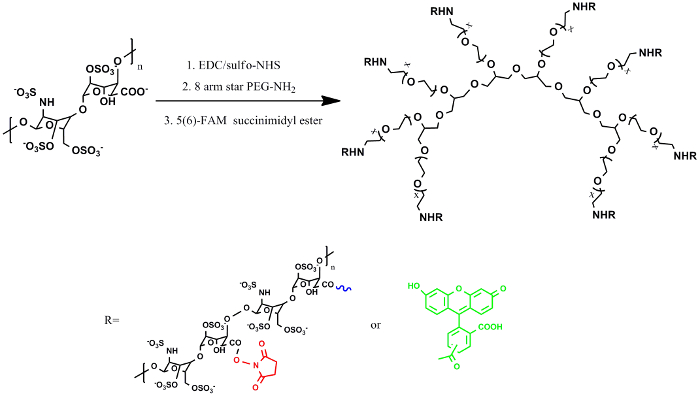
Figure 1: Chemical structure of Hep-PEG nanocoating for pancreatic islet surface engineering. Islet coating was achieved by covalent crosslinking between the heparin-NHS and primary amines within the cell membrane and star-PEG-(NH2)8. Each heparin molecule possesses multiple carboxyl groups, which were modified to have an NHS group each. The NHS-activated carboxyl groups will react with primary amines of protein to form amide linkages, via which nanocoating of Hep-PEG and islets is stabilized. Please click here to view a larger version of this figure.
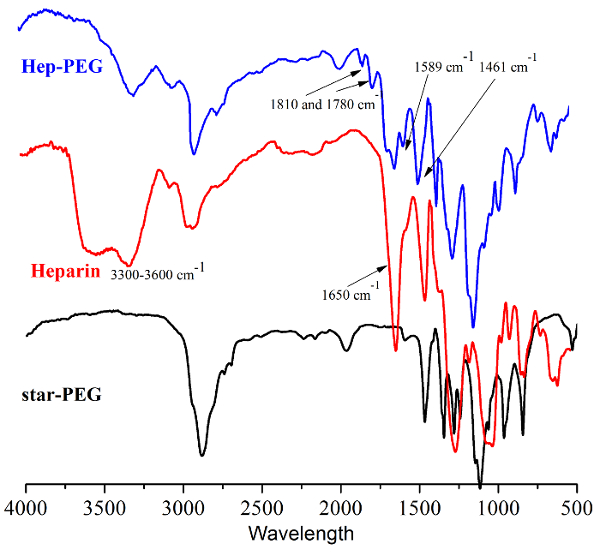
Figure 2: Infrared spectrum of star-PEG-(NH2)8, heparin and Hep-PEG nanocoating in dried state. Please click here to view a larger version of this figure.
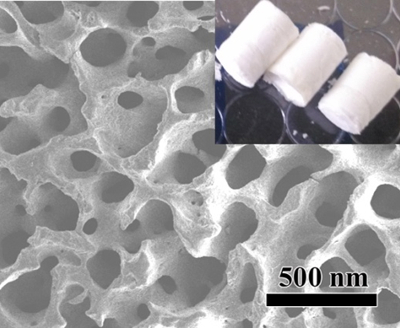
Figure 3: Scanned electronic microscopy images of Hep-PEG in dried state. Please click here to view a larger version of this figure.
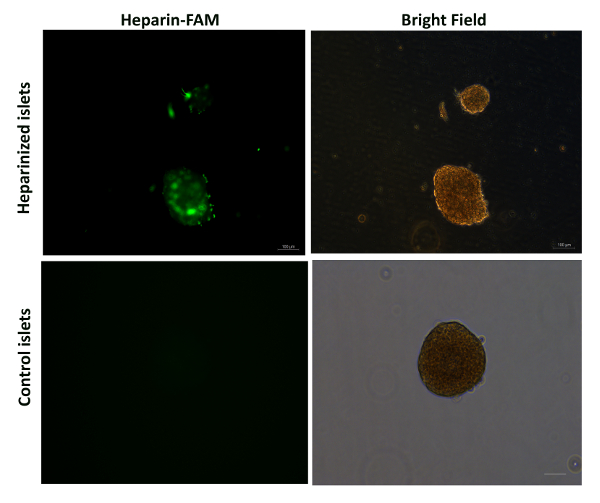
Figure 4: Representative images of heparin-PEG coated islets under fluorescence microscope.
Heparin was pre-labelled with FAM (shown in green). Images are representative of 100 islets. Scale bar = 100 µm. Please click here to view a larger version of this figure.

Figure 5: Heparin-PEG coated islets exhibited robust islet viability. (A) Data presented as mean± standard error of means, n = 50 islets per group. (B) Living cells were shown in green and dead cells in red. Scale bar = 100 µm. Images are representative of 50 islets. Please click here to view a larger version of this figure.
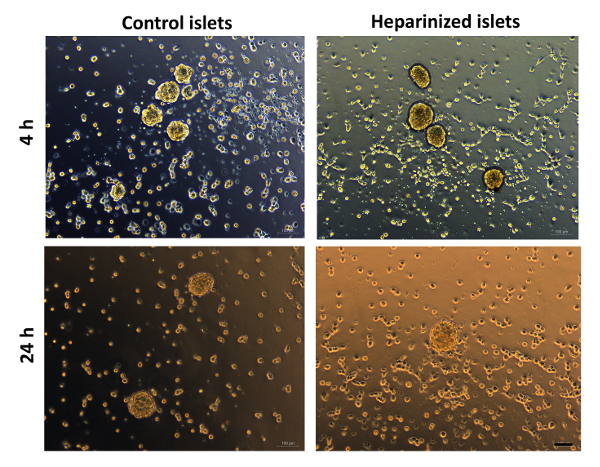
Figure 6: Heparin-PEG nanocoating facilitates intra-islet revascularisation. Matrigel tube formation of MS1 cells co-cultured with heparin-PEG coated islets and noncoated control islets. Images were taken at 4 and 24 h. Scale bar = 100 µm. Please click here to view a larger version of this figure.
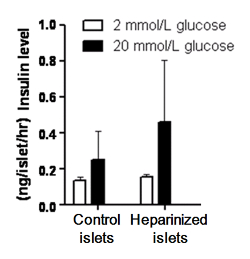
Figure 7: Heparin-PEG nanocoating incurs no change on islet insulin secretion function. Heparin-PEG coated islets and noncoated control islets (30 each) were exposed to 2 mmol/L (white bar) or 20 mmol/L (black bar) glucose for 30 min. Insulin secretion in response to 20 mmol/L glucose was comparable between the coated and control islets. Data are shown as mean ± standard error of means, n = 10. Please click here to view a larger version of this figure.
Discussion
In this article, we demonstrate an "easy-to-adopt" approach for living cell surface engineering a heparin-incorporated starPEG nanocoating via pseudo-bioorthogonal chemistry between the N-hydroxysuccinimide groups of the nanocoating and the amine groups of pancreatic islet surface membrane. Indeed, the amino groups within cell membranes are highly reactive, and as a result, earlier studies have reported interactions between primary amino groups with activated N-hydroxysuccinimidyl (NHS) ester under physiological conditions14,16,21. Furthermore, extensive research has reported that incorporation of heparin, a highly sulphated glycosaminoglycan and important component of the extracellular matrix, during islet encapsulation, could lead to enhanced post-transplantation revascularization and reduced IBMIR22,23. Considering the biocompatibility of PEG and multivalent properties of heparin, we used 8-armed PEG for maximal heparin loading during fabrication of the nanocoating. Heparin was modified with -NHS, which would subsequently react with the -NH2 groups on islet cell membrane. By enabling the covalent bond formation between -NH2 (of cell membrane) and -NHS of the Hep-PEG, the islets would be readily "coated" by the heparin-incorporated PEG, thus forming a nano-thin layer (nanocoating) on the outer surface of the pancreatic islets.
The present approach is different from previously published methods that also selected PEG as the major polymer for islet microencapsulation in that pseudo-bioorthogonal chemistry between the -NHS (of the nanocoating) and -NH2 of the islet cell membrane was used. Considering that stability of islet/cell coating, especially in a complex environment such as the plasma, is crucial to post-transplantation revascularization and survival, the formation between -NHS and -NH2 would be more stable compared to hydrophobic interaction between PEG and cell membrane24, electrostatic interactions9,15,24,25,26 or biological linkage between biotin streptavidin14.
In addition, in contrast to islet coating approach that relies on the LBL approach with extended islet handling period for multi-layer deposition14,16,25, the present technique also requires minimal processing and very short coating period of the isolated islets. Both of these factors are essential for post-transplantation islet survival since islets viability is often already compromised following islet isolation due to damaged ECM during enzymatic digestion. However, one limitation of the present approach is that, unlike LBL, via which thickness of the outer coating could be controlled by increasing or reducing the number of layer deposition, thickness of the Hep-PEG nanocoating cannot be tailored for the time being.
Furthermore, due to the mild condition where chemical reaction between -NHS and -NH2 takes place, the present approach is applicable for living cell surface engineering not limited to pancreatic islets, but most cell therapy. Additionally, considering that heparin is known to interact with a range of cytokines and biologically active molecules, the Hep-PEG nanocoating also presents an open platform that has the potential for incorporation of unlimited biological mediators as well as interfaces for more complex cell surface engineering.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
We are grateful for the financial support of the National Natural Science Funds of China (31770968) and the Tianjin Research Program of Application Foundation and Advanced Technology (17JCZDJC33400).
Materials
| Reagent | |||
| PBS | Hyclone | AAJ207798 | |
| Streptozototin | Sigma | S0130 | |
| Histopaque | Sigma | 10831 | |
| RPMI 1640 | GIBCO, by Life Technologies | 31800022 | |
| Fetal Bovine Serum | GIBCO, by Life Technologies | 16000-044 | |
| Penicillin Streptomycin | GIBCO, by Life Technologies | 15140 | |
| Cell Dissociation Solution | GIBCO, by Life Technologies | 13150-016 | |
| DMEM | GIBCO, by Life Technologies | 12800017 | |
| D-(+)-Glucose solution | Sigma | G8644 | |
| 488 phalloidin | Sigma | A12379 | |
| CFSE | Sigma | 21888-25mg-F | |
| Annexin V/PI apoptosis kit | Dojindo | AD10 | |
| DAPI Fluoromount-G | SouthernBiotech | 0100-20 | |
| Collagenase from Clostridium, Type XI | Sigma | C7657 | |
| Heparin | Sigma-Aldrich | H3149 | |
| NHS | Sigma-Aldrich | 56480 | |
| EDC | Sigma-Aldrich | 3449 | |
| 8-armed PEG | J&K Scientific Ltd | 1685176 | |
| FAM | Sigma-Aldrich | M041100 | |
| 5(6)-carboxyfluorescein N-succinimidyl ester | Sigma-Aldrich | 21888 | |
| KBr | J&K Scientific Ltd | 32036 | |
| 3-aminopropyl-triethoxysilane | Sigma-Aldrich | A3648 | |
| toluene | J&K Scientific Ltd | S-15497-20X | |
| Live/dead staining kit | Biovision, US | K501 | |
| BD MatrigelTM, basement membrane matrix, growth factor reduced | BD Bioscience | 354230 | |
| Sodium chloride, 99.5% | J&K Scientific Ltd | 105864 | |
| Potassium chloride, 99%, extra pure | J&K Scientific Ltd | 991468 | |
| Sodium bicarbonate, 99.7%, ACS reagent | J&K Scientific Ltd | 988639 | |
| Magnesium chloride hexahydrate, 99%, ACS reagent | J&K Scientific Ltd | 182158 | |
| Potassium dihydrogen phosphate, 99%, extra pure | J&K Scientific Ltd | 128839 | |
| Magnesium sulfate heptahydrate, 99%, for analysis | J&K Scientific Ltd | 119370 | |
| Calcium chloride solution volumetric, 1.0 M CaCl2 | J&K Scientific Ltd | 21114 | |
| Bovine Serum Albumin | Sigma-Aldrich | V900933 | |
| Rat/Mouse Insulin ELISA kit | Millipore-linco | EZRMI-13K |
Referencias
- Ke, T., et al. Netrin-1 ameliorates myocardial infarction-induced myocardial injury: mechanisms of action in rats and diabetic mice. Hum Gene Ther. 25 (9), 787-797 (2014).
- Ke, T., et al. Co-transplantation of skin-derived precursors and collagen sponge facilitates diabetic wound healing by promoting local vascular regeneration. Cell Physiol Biochem. 37 (5), 1725-1737 (2015).
- Saxon, E., Bertozzi, C. R. Cell surface engineering by a modified Staudinger reaction. Science. 287 (5460), 2007-2010 (2000).
- Sarkar, D., et al. Engineered cell homing. Blood. 118 (25), 184-191 (2011).
- Kato, M., Mrksich, M. Rewiring cell adhesion. J Am Chem Soc. 126 (21), 6504-6505 (2004).
- Cheng, H., et al. Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell-selectin interaction kinetics. Biomaterials. 33 (20), 5004-5012 (2012).
- Merzaban, J. S., et al. Cell surface glycan engineering of neural stem cells augments neurotropism and improves recovery in a murine model of multiple sclerosis. Glycobiology. 25 (12), 1392-1409 (2015).
- Silvescu, C. I., Sackstein, R. G-CSF induces membrane expression of a myeloperoxidase glycvariant that operates as an E-selectin ligand on human myeloid cells. Proc Natl Acad Sci U S A. 111 (29), 10696-10701 (2014).
- Zhi, Z. L., et al. Assembly of bioactive multilayered nanocoatings on pancreatic islet cells: incorporation of alpha1-antitrypsin into the coatings. Chem Commun (Camb). 51 (53), 10652-10655 (2015).
- Lou, S., et al. Pancreatic islet surface bioengineering with a heparin-incorporated starPEG nanofilm. Mater Sci Eng C Mater Biol Appl. 78, 24-31 (2017).
- Orlando, G., et al. Cell replacement strategies aimed at reconstitution of the beta-cell compartment in type 1 diabetes. Diabetes. 63 (5), 1433-1444 (2014).
- Kollmer, M., et al. Long-term function of alginate-encapsulated islets. Tissue Eng Part B Rev. 22 (1), 34-36 (2015).
- Yang, H. K., Yoon, K. H. Current status of encapsulated islet transplantation. J Diabetes Complications. 29 (5), 737-743 (2015).
- Kizilel, S., et al. Encapsulation of pancreatic islets with nano-thin functional polyethylene glycol coatings for enhanced insulin secretion. Tissue Eng. Part A. 16, 2217-2228 (2010).
- Zhi, Z. L., et al. Nano-scale encapsulation enhances allograft survival and function of islets transplanted in a mouse model of diabetes. Diabetologia. 55, 1081-1090 (2012).
- Gattas-Asfura, K. M., Stabler, C. L. Layer-by-layer deposition of antifouling coatings on stainless steel via catechol-amine reaction. ACS Appl. Mater. Interfaces. 5, 9964-9974 (2013).
- Kollmer, M., et al. Long-term function of alginate-encapsulated islets. Tissue Eng. Part B Rev. 22 (1), 34-46 (2015).
- Yang, H. K., Yoon, K. H. Current status of encapsulated islet transplantation. J. Diabetes Complicat. 29, 737-747 (2015).
- Zmuda, E. J., et al. A method for murine islet isolation and subcapsular kidney transplantation. J. Vis. Exp. (50), e2096 (2011).
- Gey, G. O., Gey, M. K. The maintenance of human normal cells and tumor cells in continuous culture. I. Preliminary report: Cultivation of mesoblastic tumors and normal tissue and notes on methods of cultivation. Am. J. Cancer. 27, 45-76 (1936).
- Chen, X., et al. Site-selective azide incorporation into endogenous RNase A via a “chemistry” approach. Org. Biomol. Chem. 11, 353-361 (2013).
- Cabric, S., et al. Anchoring of vascular endothelial growth factor to surface-immobilized heparin on pancreatic islets: implications for stimulating islet angiogenesis. Tissue Eng. Part A. 16, 961-970 (2010).
- Cabric, S., et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 56, 2008-2015 (2007).
- Asif, S., et al. Heparinization of cell surfaces with short peptide-conjugated PEG-lipid regulates thromboinflammation in transplantation of human MSCs and hepatocytes. Acta Biomater. 35, 194-205 (2016).
- Teramura, Y., et al. Microencapsulation of cells, including islets, within stable ultra-thin membrane of maleimide-conjugated PEG-lipid with multifunctional crosslinkers. Biomaterials. 34 (11), 2683-2893 (2013).
- Zhi, Z., et al. Multilayer nanoencapsulation: A nanomedicine technology for diabetes research and management. Diabetes Res. Clin. Pract. 100, 162-169 (2013).

