Dissection and Coronal Slice Preparation of Developing Mouse Pituitary Gland
Summary
We present a protocol to dissect pituitary glands and prepare pituitary coronal sections from developing mice.
Abstract
The pituitary gland or hypophysis is an important endocrine organ secreting hormones essential for homeostasis. It consists of two glands with separate embryonic origins and functions — the neurohypophysis and the adenohypophysis. The developing mouse pituitary gland is tiny and delicate with an elongated oval shape. A coronal section is preferred to display both the adenohypophysis and neurohypophysis in a single slice of the mouse pituitary.
The goal of this protocol is to achieve proper pituitary coronal sections with well-preserved tissue architectures from developing mice. In this protocol, we describe in detail how to dissect and process pituitary glands properly from developing mice. First, mice are fixed by transcardial perfusion of formaldehyde prior to dissection. Then three different dissecting techniques are applied to obtain intact pituitary glands depending on the age of mice. For fetal mice aged embryonic days (E) 17.5 – 18.5 and neonates up to 4 days, the entire sella regions including the sphenoid bone, gland, and trigeminal nerves are dissected. For pups aged postnatal days (P) 5 – 14, the pituitary glands connected with trigeminal nerves are dissected as a whole. For mice over 3 weeks old, the pituitary glands are carefully dissected free from the surrounding tissues. We also display how to embed the pituitary glands in a proper orientation by using the surrounding tissues as landmarks to obtain satisfying coronal sections. These methods are useful in analyzing histological and developmental features of pituitary glands in developing mice.
Introduction
The pituitary gland or hypophysis is an important endocrine organ secreting hormones essential for homeostasis1,2. Anatomically, the pituitary gland is a ''two-in-one'' structure consisting of the neurohypophysis and the adenohypophysis. These parts have different embryonic origins and function very differently. The neurohypophysis is derived from the neural ectoderm and secretes oxytocin and antidiuretic hormone. The adenohypophysis originates from Rathke's pouch and is responsible for the release of hormones including growth hormone, prolactin, thyroid-stimulating hormone, follicle-stimulating hormone, luteinizing hormone, adrenocorticotropic hormone, and melanocyte-stimulating hormone3,4,5.
The pituitary gland rests on the dorsal surface of the sphenoid bone (sella turcica) of the mouse skull and is attached to the floor of the brain by a fragile stalk. It is surrounded laterally by trigeminal nerves and anteriorly by the optic chiasm6,7. The gland has an elongated oval shape with its long axis perpendicular to that of the head. On its dorsal surface, the neurohypophysis and the adenohypophysis can easily be demarcated, with the former occupying the dorsal medial region and the latter extending laterally and ventrally. During postnatal development, the size of pituitary increases rapidly in the first month after birth8,9. Nevertheless, the mouse pituitary is still very small in size with an average weight of 1.9 mg and a long-axis diameter of ~3 mm in adult10, a long-axis diameter of 2 – 2.5 mm at postnatal day 21 (P21), and only 1 – 1.5 mm at P0.
A coronal section is preferred to display both adenohypophysis and neurohypophysis in a single slice of the mouse pituitary. However, some technical skills are required to obtain satisfying coronal sections of pituitary glands from developing mice due to its exceptionally small size and distinct anatomy. In this video article, we demonstrate how to dissect mouse pituitary glands and prepare pituitary coronal sections at different development stages.
Protocol
C57BL/6 mice are bred in specific pathogen-free conditions. All animal experimental methods are in compliance with the guidelines approved by the Animal Care and Ethics Committee at Second Military Medical University.
1. Dissection of Postnatal Developing Pituitary Gland
- Perform anesthesia: Place neonatal mice (P0 – P5) on crushed ice to induce hypothermia. For mice older than 5 days of age, inject 5% urethane (30 µL/g of body weight) intraperitoneally to induce anesthesia. Assess responses to tail/toe pinches. Proceed only after the mouse is unresponsive to the noxious stimuli.
- Secure the mouse in the supine position (lying on the back with face upward) by gently taping the forepaws and hindpaws to a work surface that is capable of being pinned (i.e., Styrofoam) inside a chemical fume hood.
- Perform transcardial perfusion.
- Cut the mouse ribcage with surgical scissors to expose the heart (and other thoracic organs).
- Secure the beating heart with blunt forceps and insert a 26G (0.45 mm) needle into the left ventricle. The needle is attached to a 10 mL syringe filled with heparinized phosphate buffered saline (PBS; supplemented with 5 mg/mL sodium nitrite and 10 U/mL heparin, pH 7.4, warm to 37 °C before use). Immediately cut the right atrium with fine scissors and begin to perfuse the body with the warm PBS until the fluid exiting the right atrium is clear.
NOTE: Approximate 5 – 10 mL PBS is needed for neonatal mice and 10 – 20 mL PBS for adult mice. - Keep the needle in place and change to another syringe filled with 4% paraformaldehyde (PFA; pH 7.4). Perfuse 10 mL and 20 mL of fixative for neonatal and P21 mouse, respectively.
Caution: PFA is toxic. It may cause skin and respiratory irritations, and eye damage. Wear gloves and work under a chemical hood.
- Decapitate the PFA-fixed mouse and cut the skull bone open with scissors.
- Gently lift the hindbrain from the base of the skull with small forceps. At the first sign of the sella turcica, stop lifting but hold the hindbrain, cut the pituitary stalk and nerve fibers connecting to the base of the brain with fine scissors. This step is critical to ensure that the pituitary is not lifted away with the brain.
- Then keep lifting the brain and remove the whole brain to expose the pituitary gland fully. Cut the entire sella region including the pituitary gland, lateral trigeminal nerves, and the beneath sphenoid bone with scissors (approximately 3 x 5 mm2).
- Put the dissected tissue into a 35 mm dish or one well of a 6-well plate containing 2 mL cold 4% PFA (pH 7.4). Fix the tissue at 4 °C for 40 min for P0 – P7 pituitaries, 1 h for P14 pituitaries, 1.5 h for P21 – P28 pituitaries, and 3 h for adult pituitaries.
- Further dissociation of pituitary
- Wash the fixed tissue with 10 mL PBS, 5 changes, 15 min each. The neonatal pituitary gland together with its surrounding tissues and the beneath sphenoid bone can be processed directly to dehydration. However, pituitary glands from mice older than 5 days should be dissociated further under a stereo microscope.
- Put the fixed tissue into a 35 mm dish containing 1 mL PBS. For P5 – P14 pituitaries, remove the connective membranes between the nerves and the bone with fine forceps and scissors, and carefully isolate the pituitary gland together with lateral trigeminal nerves as a whole but leaving the sella turcica.The isolated gland and nerves are connected by connective membranes with a "H"-like appearance (Figure 1B).
- For P21 and adult pituitaries, remove the nerves and connective membranes around the pituitary and free the gland from the surrounding tissues. Wrap the pituitary tissue with lens cleaning tissue paper and put it into an embedding cassette.
NOTE: Wrapping the tiny pituitary with lens cleaning tissue paper helps to prevent any potential loss of specimens and preserve tissue integrity in the following paraffin-embedding procedure. It may not be necessary to wrap the pituitary if it is processed in a small bottle in the cryo-embedding procedure.
2. Paraffin Embedding and Sectioning
- Dehydrate the specimens in embedding cassettes with 50%, 65%, 75%, and 95% ethanol solutions for 15 min each. Subsequently dehydrate with 3 changes of pure ethanol, 3 min each for P0 – P4 glands, 4 min each for P5 – P14 glands, and 5 min each for P21 and older glands. Clear with xylene, 3 changes, 3 min each for P0 – P4 glands, 4 min each for P5 – P14 glands, and 5 min each for P21 and older glands. Infiltrate with molten paraffin wax, 3 changes, 7 min each for P0 – P4 glands, and 8 min twice followed by 6 min once for P5 and older glands.
NOTE: The optimal parameters for tissue processing can be empirically adjusted. To obtain high quality sections, insufficient or over dehydration should be avoided. The same is true for clearing the tissue using xylene. - Orientation when embedding
- On a tissue embedding console system, remove the specimen from the cassette and place it into a base mold half-filled with molten paraffin wax. Proper orientation of the embedding pituitary gland is essential for satisfying coronal sections.
- For P21 and adult pituitary glands, position the pituitary gland with its short axis perpendicular to the bottom surface of a base mold (the section plane). Use sphenoid bones and trigeminal nerves as anatomic landmarks for P0 – P4 and P5 – P14 pituitaries, respectively. Orient specimens with their trigeminal nerves or transversal lamina of sphenoid bones perpendicular to the bottom surface of a base mold.
- Gently hold the tissue in the desired position with warmed fine forceps until the wax becomes semi-solid on a cooling plate. Top up the mold with molten paraffin wax. Allow the paraffin block to cool until the wax is fully solidified.
NOTE: The pituitary at embryonic day 18.5 (E18.5) can be treated in the same way as the neonatal pituitary. But the pituitaries (Rathke's pouches) younger than E16.5 should be embedded with the entire head and oriented with the sagittal axis (from mouth to occiput) perpendicular to the bottom surface of a base mold.
- Chill the paraffin block at -20 °C for 10 – 20 min and then cut it into thin slices (4 µm thickness is preferred) with a microtome. To get satisfactory coronal sections, fine tune the position of paraffin blocks during sectioning. Mount the tissue slices onto polylysine-coated glass slides to prevent detachment of slices in the following procedures.
NOTE: Alternatively, specimens can be dehydrated in 15%, 30% (W/V) sucrose solutions (in PBS) at 4 °C until they sink to the bottom, embedded in cryo-embedding compound using the same orientation method, and quickly frozen on dry ice. Then cut the frozen tissue blocks into slices of 8 – 10 µm using a cryostat. The morphology of cryo-sections, however, is often inferior to paraffin sections.
3. H&E Staining and Immunofluorescence Labeling
- Warm the slides on a heat block at 55 °C and dewax with xylene, 2 changes, 4 min each. Dehydrate the samples with 95% ethanol twice and 75% ethanol twice, 3 min each. Wash the slides with distilled water for 5 min on a shaker.
- For H&E staining, stain the sections in alum hematoxylin solution for 6 min, rinse in running tap water for 2 min, differentiate with 0.2% ammonia water for 45 s, and re-rinse in running water for 2 min. Then dehydrate the sections with 95% ethanol for 1 min and stain with eosin for 2 min. Sequentially dehydrate, clear, and mount.
- Immunofluorescence labeling
- Treat the sections with methanol containing 3% H2O2 and 0.01% NaN3 for 20 min at room temperature to inactivate endogenous peroxidases. Retrieve antigens by boiling sections in the retrieval buffer (1 mM EDTA and 1 mM Tris-HCl, pH 7.4) in a pressure cooker for 2 min. Incubate the sections with blocking buffer (5% goat serum in PBS) for 30 min at 37 °C.
- Incubate sections with rabbit anti-growth hormone (GH) antibody (1:2,000) or rabbit anti-glial fibrillary acidic protein (GFAP) antibody (1:2,000) diluted in blocking buffer overnight at 4 °C.
- Incubate sections with secondary antibody (goat anti-rabbit IgG-HRP, 1:300 in blocking buffer) for 35 min at 37 °C.
- Amplify the signals using Biotinyl Tyramide (1:50 in Tris buffered saline containing 0.3% H2O2) for 15 min at room temperature, and visualize with Streptavidin-Alexa594 or 488 (1:300 in blocking buffer, 30 min, 37 °C).
- Counterstain the nuclei with DAPI (50 mg/L in PBS) for 5 min at room temperature.
NOTE: It may not be necessary to amplify the signal using Tyramide Signal Amplification (TSA) system. Alternatively, a fluorescence-conjugated secondary antibody can be used to visualize the signal.
- Acquire images using a fluorescence microscope equipped with DAPI, Texas Red, and FITC filter sets, ×4/0.13 to ×40/0.75 objectives and 5.0 megapixel camera. Use the 360, 488, and 594 nm laser wavelengths for imaging DAPI, Alexa 488-, and Alexa 594-stained sections, respectively. Optimize the laser power (0.8 was used generally) and exposure time to the desired levels for each channel. Capture the image under the control of image acquisition software and overlay the fluorescence images subsequently using image processing software.
Representative Results
This protocol presents a method to dissect pituitary glands from developing mice. For the neonatal mouse, the whole sella regions containing the pituitary gland, the trigeminal nerves, and the underneath sphenoid bone were dissected out from the skull base. The tiny and delicate pituitary gland remained intact during the process (Figure 1A). For mice older than 5 days, the pituitary glands attached to the lateral trigeminal nerves were then isolated. The gross structure of the pituitary gland isolated from the P7 mouse was well preserved (Figure 1B). For the P21 mouse, the size of pituitary gland was increased remarkably compared to that of the neonatal mouse. The pituitary gland was successfully isolated with invisible damage while removing its surrounding tissues (Figure 1C).
To view the maximum region of both adenohypophysis and neurohypophysis in a single slice of the pituitary, a coronal section is preferred. The dissected pituitary glands were properly oriented to achieve satisfying coronal sections. H&E staining showed well preserved morphology of both adenohypophysis and neurohypophysis in P0, P7, and P21 pituitary glands (Figure 2).
The processed slices were also compatible with immunofluorescence labeling. As an example, the adenohypophysis and the neurohypophysis showed specific immunolabeling of GH and GFAP, respectively (Figure 3).
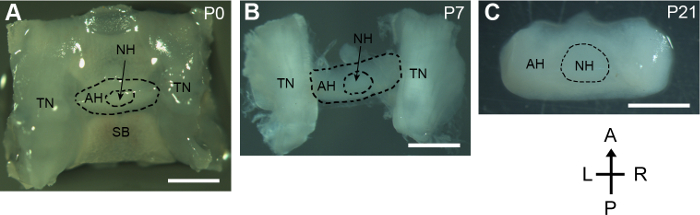
Figure 1: Dissection of postnatal developing pituitary glands. Dorsal views of dissected pituitary glands and surrounding tissues from P0, P7, and P21 mice. A, anterior; P, posterior; L, left; R, right; SB, sphenoid bone; TN, trigeminal nerve; AH, adenohypophysis; NH, neurohypophysis. Scale bars, 1 mm. Please click here to view a larger version of this figure.
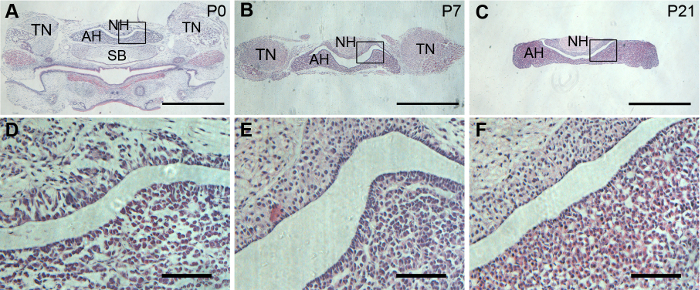
Figure 2: Histology of mouse pituitary glands. Representative H&E staining on pituitary coronal sections from P0, P7, and P21 mice. (A, B, and C) are low magnification views of coronal pituitaries. (D, E, and F) are enlargement of the framed areas in (A, B, and C) respectively. Scale bars = 1 mm in (A, B, and C); and 20 µm (D, E, and F). Please click here to view a larger version of this figure.

Figure 3: Representative immunofluorescent staining on pituitary glands. (A) GH (red) was specifically expressed in the adenohypophysis from P7 mice. (B) Magnified image of the framed area in A. (C) GFAP (green) was specifically expressed in the neurohypophysis from P21 mice. (D) Magnified image of the framed area in C. Nuclei were stained with DAPI (blue). Scale bar = 300 µm (A and C); and 30 µm (B and D). Please click here to view a larger version of this figure.
Discussion
For developing murine pituitaries, it has been technically difficult to obtain proper coronal sections due to their tiny and fragile features and unique anatomical characteristics6,8. Some research groups thus chose mid-sagittal sections to analyze the morphology of embryonic and neonatal pituitary11,12. Though the mid-sagittal section of pituitary is also capable of showing anterior, intermediate, and posterior lobes in a single slice, the coronal section is highly preferred as it can show a maximum view of these three lobes. In particular, for quantification studies, such as determination of the volume and area of the pituitary gland and counting the number of apoptotic and proliferating cells, coronal sections are superior to mid-sagittal sections in terms of their representative. Here, we present a method useful to dissect pituitary glands and prepare pituitary coronal sections from developing mice.
To avoid damaging the pituitary glands during the process, several techniques have been used in this protocol. First, mice are fixed by transcardial perfusion of formaldehyde prior to dissection. This fixative not only helps to preserve the organ architecture but also to remove red blood cells which often result in auto fluorescence13. Second, the entire sella region is dissected to avoid touching the pituitary gland with any hard-surgical tools. The surrounding tissues help to keep the pituitary gland in its original position and also serve as landmarks for embedding orientation. With these methods employed, smaller postnatal pituitaries from younger animals can be dissected whilst reducing the probability of undesired damage.
The dissection procedure in this protocol is also applicable to isolate murine pituitary that has not been pre-fixed by perfusion. In that case, more caution should be taken to avoid any undesired damage and the gland should also be dissected as quickly as possible.
Since developing murine pituitaries are very small in size, it has been very difficult to orientate them properly when embedding. This protocol uses the trigeminal nerves or sphenoid bones as landmarks, making the orientation steps much easier. Properly oriented pituitaries are essential for achieving satisfying coronal sections.
In summary, we provide an improved pituitary dissection and embedding protocol, which is suitable for mice at different development stages. Application of these procedures can obtain satisfying coronal sections of murine pituitary glands to be used for analysis of routine histology, immunohistochemistry, in situ hybridization, and developmental research14.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the grants from National Natural Science Foundation of China (31201086, 31470759 and 31671219) and Shanghai Natural Science Foundation (12ZR1436900).
Materials
| Tools/Equipment | |||
| Surgical scissors-straight | JinZhong | J21010 | can be purchased from other vendors |
| Fine scissors-strainght | JinZhong | WA1010 | can be purchased from other vendors |
| Blunt forceps | JinZhong | JD1020 | can be purchased from other vendors |
| Fine forceps | Dumont | RS-5015 | for isolation of the pituitary |
| 26G (0.45mm) needle | HongDa | for transcardial perfusion | |
| Syringe (1 mL) | BD | 300841 | can be purchased from other vendors |
| Syringe (10 mL) | BD | can be purchased from other vendors | |
| 35mm dish | Corning | 430165 | can be purchased from other vendors |
| Lens cleaning paper | ShuangQuan | can be purchased from other vendors | |
| Anatomical microscope | OLYMPUS | SZX-ILLB2-200 | can be purchased from other vendors |
| Embedding cassette | Thermo Fisher | 22-272423 | can be purchased from other vendors |
| Tissue embedding console system | KEDEE | KD-BM11 | can be purchased from other vendors |
| Microtome | Thermo Fisher | HM315R | can be purchased from other vendors |
| Superfrost-Plus slides | Thermo Fisher | 22-037-246 | can be purchased from other vendors |
| Cover glass | Thermo Fisher | 12-543 | can be purchased from other vendors |
| Fluorescence microscope | OLYMPUS | BH2-RFCA | can be purchased from other vendors |
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| Urethane | BBI | EB0448 | |
| NaCl | Sigma | S9625 | for PBS |
| KCl | Sigma | P9541 | for PBS |
| Na2HPO4.12H2O | Sigma | 71650 | for PBS |
| K2HPO4 | Sigma | P2222 | for PBS |
| NaNO2 | Sigma | 237213 | |
| Heparin Sodium Injection | SPH | H31022051 | for perfusion saline |
| Paraformaldehyde (PFA) | Sigma | P6148 | |
| Ethanol | SCR | 10009218 | |
| Xylene | SCR | 10023418 | |
| Paraffin | Thermo Fisher | 8330 | |
| Hematoxylin | Sigma | H9627 | for H&E staining |
| Eosin Y | Sigma | E4009 | for H&E staining |
| rabbit anti growth hormone (GH) | National Hormone | for immunostaining | |
| antibody | Pituitary Program | ||
| Rabbit anti-mouse GFAP antibody | Sigma | G9269 | for immunostaining |
| Goat anti-rabbit IgG, HRP | Jackson | 111-035-003 | for immunostaining |
| TSA system | NEN Life Science Products | NEL700 | for immunostaining |
| Streptavidin, Alexa Fluor 594 | Thermo Fisher | S32356 | for immunostaining |
| Anti-FITC Alexa Fluor 488 | Thermo Fisher | A11090 | for immunostaining |
Referencias
- Fauquier, T., Lacampagne, A., Travo, P., Bauer, K., Mollard, P. Hidden face of the anterior pituitary. Trends Endocrinol Metab. 13 (7), 304-309 (2002).
- Haedo, M. R., et al. Regulation of pituitary function by cytokines. Horm Res. 72 (5), 266-274 (2009).
- Zhu, X., Gleiberman, A. S., Rosenfeld, M. G. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 87 (3), 933-963 (2007).
- Bancalari, R. E., Gregory, L. C., McCabe, M. J., Dattani, M. T. Pituitary gland development: an update. Endocr Dev. 23, 1-15 (2012).
- Kelberman, D., Rizzoti, K., Lovell-Badge, R., Robinson, I. C., Dattani, M. T. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 30 (7), 790-829 (2009).
- Peker, S., Kurtkaya-Yapicier, O., Kilic, T., Pamir, M. N. Microsurgical anatomy of the lateral walls of the pituitary fossa. Acta Neurochir (Wien). 147 (6), 641-648 (2005).
- Wolpert, S. M., Molitch, M. E., Goldman, J. A., Wood, J. B. Size, shape, and appearance of the normal female pituitary gland. AJR Am J Roentgenol. 143 (2), 377-381 (1984).
- Carbajo-Perez, E., Watanabe, Y. G. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 261 (2), 333-338 (1990).
- Nantie, L. B., Himes, A. D., Getz, D. R., Raetzman, L. T. Notch signaling in postnatal pituitary expansion: proliferation, progenitors, and cell specification. Mol Endocrinol. 28 (5), 731-744 (2014).
- Tzou, S. C., Landek-Salgado, M. A., Kimura, H., Caturegli, P. Preparation of mouse pituitary immunogen for the induction of experimental autoimmune hypophysitis. J Vis Exp. (46), (2010).
- Budry, L., et al. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc Natl Acad Sci U S A. 108 (30), 12515-12520 (2011).
- Wilson, D. B., Wyatt, D. P. Histopathology of the pituitary gland in neonatal little (lit) mutant mice. Histol Histopathol. 7 (3), 451-455 (1992).
- Jonkers, B. W., Sterk, J. C., Wouterlood, F. G. Transcardial perfusion fixation of the CNS by means of a compressed-air-driven device. J Neurosci Methods. 12 (2), 141-149 (1984).
- Cao, D., et al. ZBTB20 is required for anterior pituitary development and lactotrope specification. Nat Commun. 7, 11121 (2016).

