Light-mediated Formation and Patterning of Hydrogels for Cell Culture Applications
Summary
We describe a sequential process for light-mediated formation and subsequent biochemical patterning of synthetic hydrogel matrices for three-dimensional cell culture applications. The construction and modification of hydrogels with cytocompatible photoclick chemistry is demonstrated. Additionally, facile techniques to quantify and observe patterns and determine cell viability within these hydrogels are presented.
Abstract
Click chemistries have been investigated for use in numerous biomaterials applications, including drug delivery, tissue engineering, and cell culture. In particular, light-mediated click reactions, such as photoinitiated thiol−ene and thiol−yne reactions, afford spatiotemporal control over material properties and allow the design of systems with a high degree of user-directed property control. Fabrication and modification of hydrogel-based biomaterials using the precision afforded by light and the versatility offered by these thiol−X photoclick chemistries are of growing interest, particularly for the culture of cells within well-defined, biomimetic microenvironments. Here, we describe methods for the photoencapsulation of cells and subsequent photopatterning of biochemical cues within hydrogel matrices using versatile and modular building blocks polymerized by a thiol−ene photoclick reaction. Specifically, an approach is presented for constructing hydrogels from allyloxycarbonyl (Alloc)-functionalized peptide crosslinks and pendant peptide moieties and thiol-functionalized poly(ethylene glycol) (PEG) that rapidly polymerize in the presence of lithium acylphosphinate photoinitiator and cytocompatible doses of long wavelength ultraviolet (UV) light. Facile techniques to visualize photopatterning and quantify the concentration of peptides added are described. Additionally, methods are established for encapsulating cells, specifically human mesenchymal stem cells, and determining their viability and activity. While the formation and initial patterning of thiol-alloc hydrogels are shown here, these techniques broadly may be applied to a number of other light and radical-initiated material systems (e.g., thiol-norbornene, thiol-acrylate) to generate patterned substrates.
Introduction
Click chemistries are increasingly used in the design of materials for numerous biomedical applications, including drug delivery, tissue engineering, and controlled cell culture, owing to their selective, efficient, and often cytocompatible reactivities.1-3 Photoclick chemistries that utilize light to trigger or initiate reactions (e.g., azide-alkyne,4 thiol−ene,5 and tetrazole-alkene6) are of particular interest for the formation or modification of biomaterials. Rapid rates under mild conditions and control of when and where they take place with light make these reactions well-suited for user-directed control of biomaterial properties in the presence of cells.7,8 In particular, thiol−ene photoclick chemistries have been used to generate hydrogel-based biomaterials with robust mechanical properties5,9 and for the encapsulation of a wide variety of cell types, including, but not limited to, human mesenchymal stem cells (hMSCs), fibroblasts, chondrocytes, and pancreatic cells, with promise for cell culture and delivery.10,11 Further, these chemistries have been used for the spatial patterning of biochemical cues to mimic key aspects of native cell microenvironments and facilitate appropriate cell-matrix interactions, including adhesion, differentiation, and invasion.3,12
For the construction of thiol−ene hydrogels with light, peptides containing cysteines (thiol) commonly are reacted with polymers functionalized with acrylates or norbornenes ('ene') for rapid, photoinitiated polymerization under cytocompatible conditions.13 Expanding this toolbox, we sought to establish methods for hydrogel formation with new versatile and accessible building blocks that required minimal synthetic processing or were commercially available toward their broad use as synthetic extracellular matrices.14 Specifically, peptides were modified with allyloxycarbonyl (Alloc)-protected lysines: one for pendant, integrin-binding groups to promote cell adhesion [K(alloc)GWGRGDS = Pep1Alloc] or two for non-degradable or cell-degradable crosslinks [K(alloc)RGKGRKGK(alloc)G or KK(alloc)GGPQGIWGQGK(alloc)K = Pep2Alloc, respectively]. With these sequences, conditions were established for rapid reaction (1-5 min) with four-arm thiol-modified poly(ethylene glycol) (PEG4SH) using cytocompatible doses of long wavelength UV light (10 mW/cm2 at 365 nm) and the photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP). The resulting hydrogels were stable under cell culture conditions for weeks. To enable cell-driven degradation and remodeling, an enzymatically-cleavable peptide was incorporated within the gel crosslinks (i.e., GPQGIWGQ),15 and a model primary cell, human mesenchymal stem cells (hMSCs), remained highly viable after encapsulation and during culture within these matrices. Further, peptides have been spatially patterned within these materials, and hMSCs remain viable and metabolically active under photopatterning conditions. Alternate pendant peptide sequences, not shown here (e.g., IKVAV, YIGSR, GFOGER, etc.), also may be incorporated within matrices to probe additional cell interactions with the surrounding microenvironment. These results are promising for the application of these hydrogel-based materials for three-dimensional (3D) cell culture and delivery to study and direct cell-matrix interactions for a variety of cell types.
Herein, methods to photoencapsulate cells and subsequently photopattern biochemical cues within the proposed hydrogel system are presented (Figure 1). Techniques to observe and quantify these photopatterns also are demonstrated: notably, i) the quantitative and qualitative use of Ellman's assay to determine the modification of free thiols within patterned substrates and ii) the complementary qualitative use of fluorescent peptides (AF488Pep1Alloc) to observe these patterns in three dimensions. Further, assays to determine viability (live/dead viability/cytotoxicity staining) and metabolic activity (MTS; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) are presented so that users may determine the cytocompatibility of photoencapsulation and photopatterning conditions for different cell lines within hydrogel matrices. While the protocol is demonstrated for a facile light-based photoclick hydrogel system, the techniques may be applied to numerous other radically-initiated hydrogel systems for photoencapsulation and photopatterning in the presence of cells.
Protocol
1. Preparation of Materials for Hydrogel Formation
- Synthesize pendant (Pep1Alloc, AF488Pep1Alloc) and crosslinking peptides (Pep2Alloc) by standard solid phase peptide synthesis (SPPS) techniques and thiol-functionalized polymer by three-step procedure for end group modification (PEG4SH).14,16 Alternatively, purchase PEG4SH (Mn ~ 20 kDa), Pep1Alloc, and Pep2Alloc commercially.
- Synthesize the photoinitiator (LAP) by the two-step reaction described below.14,17 Perform synthesis steps (1.2.1-1.2.11) in a fume hood and use caution when handling chemicals (wear protective gloves, clothing, and eyewear). LAP also may be purchased commercially.
- Dry all glassware in oven (>2 hr, 80 °C).
- Add a stir bar to an empty single-neck round-bottom flask (100 ml) and cover with a septum.
- Secure the flask on top of a magnetic stirring hot plate with a ring stand and clamp.
- Insert a needle (18 G) through the septum and leave the outer end open to the atmosphere. Insert a second needle attached to an inert gas line. Open the inert gas line (e.g., argon or nitrogen) and purge the flask for 10-15 min.
NOTE: The system will be continuously purged with inert gas, argon or nitrogen, throughout the reaction. - Transfer 1.5 g (~1.4 ml) dimethyl phenylphosphonite (CAUTION) to the flask, using a syringe with needle to pierce through the septum. Turn on the stir plate (medium speed) and be careful that the contents do not splash onto the sides of the flask.
- Add 1.6 g (~1.46 ml) 2,4,6-trimethylbenzoyl chloride (CAUTION) dropwise to the flask containing dimethyl phenylphosphonite, using a syringe with a needle to pierce the septum.
- Cover the reaction vessel with foil to protect from light and stir for 18 hr under argon or nitrogen.
- The next day, raise the height of the flask, place an oil bath on the stirrer, and carefully lower the flask into the oil bath. Heat the bath and the flask to 50 °C while maintaining magnetic stirring.
- Dissolve 3.05 g lithium bromide in 50 ml of 2-butanone. Raise the flask out of the oil bath and add the lithium bromide solution to the round-bottom flask, briefly removing the septum to pour into the flask.
- Seal the flask again with the septum (CAUTION: Septum will still have a needle leading to the argon lines and a needle to vent), lower the flask back into heated oil bath, and allow the reaction to proceed for 10 min. A solid precipitate will form.
- After 10 min, turn off argon, remove the flask from heat, and allow the mixture to rest for 4 hr (covered with foil to protect from light as a light-sensitive initiator has been produced. Keep the vent needle place.
- Pour product into a glass frit funnel or funnel lined with appropriate filter paper. Rinse filtrate 3 times with 50 ml of 2-butanone to remove any unreacted lithium bromide.
- Dry (first on benchtop and then in vacuum desiccator) and analyze product by 1H NMR in D2O. Characteristic peaks at 1.8-1.9 (6H, s), 2.1-2.2 (3H, s), 6.7-6.8 (2H, s), 7.3-7.4 (2H, m), 7.4-7.5 (1H, m), and 7.5-7.7 (2H, m).14,17
- Use a spreadsheet to calculate the volume and concentration of each stock solution (PEG4SH, Pep1Alloc, Pep2Alloc, LAP) that must be prepared to make hydrogels (Table 1). To make non-patterned gels, ensure that [SH] = [Alloc] so that all reactive groups are consumed during polymerization. If photopatterning of gels is planned, include an excess amount of thiol functional groups during gel formation based on stoichiometry (e.g., 0.2-5 mM, [SH] >[Alloc]) for later reaction with Pep1Alloc.
NOTE: Gels should contain greater than 5 weight percent (wt%) PEG4SH to ensure rapid polymerization (within 5 min). However, lower wt% ranges may be explored if the application calls for hydrogels with lower moduli (e.g., <0.5 kPa); polymerization should be checked and adjusted accordingly for these low wt% compositions. Similarly, Pep1Alloc concentration may be adjusted for different applications (e.g., 0.2-5 mM) as reported in literature for thiol-functionalized peptides.18-21 LAP concentration is recommended around 0.067 wt% (2.2 mM) or less, as described, as higher concentrations can decrease cell viability. - Prepare stock solutions of Pep1Alloc, Pep2Alloc, PEG4SH, and LAP under sterile conditions for cell culture based on calculations in Table 1.
- For Pep1Alloc and Pep2Alloc, individually weigh the total mass of Pep1Alloc and Pep2Alloc from the peptide synthesis and dissolve in sterile phosphate buffered saline (PBS) containing 1% penicillin/streptomycin (PS) and 0.5 µg/ml fungizone (FZ). Typical concentrations of prepared stock solutions range from 20-100 mg/ml of peptide. Mix these stocks to achieve gels with final moduli relevant for soft tissue applications (Young's modulus ~ 0.5-5 kPa).22,23 Aliquot and store at -20 °C until use.
- For PEG4SH, weigh PEG4SH into a sterile microcentrifuge tube and dissolve in PBS+PS+FZ. Typical concentrations of this stock solution range from 250-430 mg/ml (20-30% PEG4SH by weight).
- For LAP, weigh LAP into a sterile microcentrifuge tube and dissolve in PBS+PS+FZ to a final concentration of 7.5 mg/ml.
NOTE: Preparing fresh solutions of PEG4SH and LAP is recommended for every encapsulation or gel experiment to ensure consistent polymerization times.
- Prepare Sterile Syringe Tip Molds for Forming Hydrogels.
- Carefully cut the tips off of 1 ml syringes using a razor blade and subsequently remove the plungers from the syringe shafts.
- Submerge the syringe shafts and plungers in 70% ethanol for 15 min and place in a sterile cell culture hood to dry (30 min). If excess drops of ethanol remain inside syringe shaft, use the plunger to push them out.
- Prepare Sterile Glass Slide Molds for Forming Hydrogels.
- Soak glass slides (multiples of 2) and rubber gaskets (0.254 mm thick; 2 small pieces used as shims, a rectangle with discs punched out, or square frame shape) in 70% ethanol for 15 min, and place in sterile cell culture hood to dry.
- Coat half of the sterilized slides with anti-adhesive coating per manufacturer's directions to prevent adhesion of the top of the gel to the slide surface (e.g., if there are 4 slides, 2 slides will be coated with anti-adhesive). These will serve as the top of the glass slide mold.
- Calibrate the lamp for syringe or glass slide molds.
NOTE: For these experiments, a mercury arc lamp with an external filter adaptor assembly and 365 nm external filter was used. Other lamps that produce appropriate intensities of long wavelength UV light may be used as reported by others.24-27- Attach a collimating lens to the end of the liquid-filled light guide to ensure relatively even light intensity across all samples. As needed, adjust the distance of the light guide from the sample(s) to achieve a spot size that will cover samples of interest.
- Place a cut syringe mold in a microcentrifuge tube holder (to hold syringe mold in vertical position). Hold the radiometer at the height of the syringe tip where the samples will be held and adjust shutter (% open) to achieve a light intensity of 10 mW/cm2 at 365 nm. Record the adjusted settings for later use.
- Place a glass slide mold onto the top of a sterile surface (e.g., the top of a pipette tip box within a biosafety cabinet). Hold the radiometer detector at the height of the glass slide mold and adjust shutter (% open) to achieve a light intensity of 10 mW/cm2 at 365 nm. Record the adjusted settings for later use.
2. Hydrogel Formation and Photopatterning
- Preparation of Non-patterned Hydrogels.
NOTE: At this point in the protocol, if hydrogels are to be used in cell culture applications, all subsequent steps should be performed under sterile conditions in a sterile cabinet or hood.- Mix stock solutions of PEG4SH, Pep2Alloc, Pep1Alloc, LAP, and PBS+PS+FZ according to the spreadsheet calculation (see Table 1 example). Pipette the resulting gel precursor solution vigorously to ensure even mixing of the solution.
- Prepare thick hydrogels molded in syringe tips by pipetting 10-20 µl of the gel precursor solution (PEG/Pep/LAP/PBS) into the tip of a sterile, cut syringe. Make a single gel per syringe, approximately 0.5-1.5 mm thick based on volume and syringe diameter.
NOTE: Larger volumes may be explored based on the desired gel size where upper limits may exist based on diffusional limitations for Pep1Alloc during photopatterning and/or nutrients/wastes to/from encapsulated cells during cell culture. - Prepare thin hydrogels in glass slide molds by placing the rubber gasket (0.254-mm thick) around the edges of the non-coated glass slide. Pipette 5-10 µl gel precursor solution (single or multiple 5-10 µl gels may be made by pipetting one or more dots of solution onto a single slide) onto the non-coated glass slide and place the glass slide coated with anti-adhesive on top of the gel solution (larger gels, up to the size of the glass slide, may be made depending on the final application). Secure the glass slides with small binder clips to stabilize.
- Place molds under the lamp and set the lamp intensity (e.g., % shutter open) to achieve 10 mW/cm2 at the gel surface for syringe tip molds or glass slide molds based on measurements in step 1.7.2 and 1.7.3, respectively.
- Apply light for 1 to 5 min to allow complete polymerization of gels. Use a shorter polymerization time for gels with higher PEG4SH content (8 wt% or greater, 1 min) and longer for gels with lower PEG4SH content (5-8 wt%, 3-5 min) to produce fully-polymerized hydrogels with moduli within the range of soft tissues (0.5-5 kPa).
- Place gels from syringe tip molds into a sterile non-treated 48-well plate, and place glass slides into a sterile dish.
- Rinse 3 x 15 min with cell culture medium or appropriate buffer (e.g., PBS+PS+FZ, Ellman's reaction buffer) based on planned experiments, as detailed below.
- Preparation of Patterned Hydrogels.
- Mix stock solutions of PEG4SH, Pep2Alloc, LAP, and PBS+PS+FZ according to the spreadsheet calculation, leaving free thiols (0.2-5 mM) for later reaction with Pep1Alloc. Pipette the precursor solution vigorously to ensure even mixing of the solution.
- Prepare thick and thin hydrogels as laid out in steps 2.1.2 to 2.1.6.
NOTE: Do not place gels in growth medium prior to photopatterning. Free thiols may be consumed by species in the growth medium (e.g., disulfide formation with thiol-containing proteins) and will not allow patterning of the gel without additional processing steps (e.g., reduction of disulfide bonds). - Prepare solutions of Pep1Alloc (final concentration ~3-20 mg/ml) and 2.2 mM LAP.
- Cover pre-formed gels with Pep1Alloc/LAP solution and incubate for 1 hr at 37 °C.
- Remove excess Pep1Alloc/LAP solution. If gels were molded in a syringe tip, use a spatula to carefully transfer the gels from the 48-well plate in which they are incubating to a sterile glass slide for patterning.
- Place a photomask with the desired pattern directly on top of syringe- and glass slide-molded gels. Ensure that the printed part of the mask touches the gel for optimal pattern fidelity (i.e., mask should read right and be emulsion side down). Place samples under the lamp and irradiate for 1 min with the lamp settings used for glass slides (step 1.7.3).
- After patterning, place syringe-molded gels into a sterile, 48-well non-tissue culture treated plate, and place glass slide-molded gels that are adhered to the glass slides into a sterile dish.
- Rinse 3 x 15 min with cell culture medium or appropriate buffer (e.g., PBS+PS+FZ, Ellman's reaction buffer) based on planned experiments.
3. Visualizing and Quantification of Photopatterning
- Ellman's Assay to Detect and Quantify Free Thiols in Photopatterned Hydrogels.
- Prepare Ellman's reaction buffer, cysteine working solution, standards, and Ellman's reagent as described below.
- For Ellman's Reaction Buffer, dissolve 2.4 g sodium phosphate dibasic (Na2HPO4) and 74.4 mg ethylenediaminetetraacetic acid (EDTA) in 200 ml dIH2O (0.1 M Na2HPO4, 1 mM EDTA). Adjust the pH to 7.5-8 with solutions of sodium hydroxide (NaOH) or phosphoric acid (H3PO4).
- For Cysteine Working Solution, dissolve 5.27 mg cysteine in 15 ml reaction buffer (2 mM cysteine).
- For Cysteine Standards, dilute cysteine working solution in reaction buffer to a final concentration of 2, 1.5, 1.25, 1, 0.75, 0.5, 0.25, and 0 mM cysteine.
- For Ellman's Reagent, dissolve 4 mg Ellman's reagent in 1 ml reaction buffer. Sonicate to completely dissolve the Ellman's reagent.
- Quantify the concentration of free thiols in gels with Ellman's assay (Figure 2).
NOTE: 'Thin' 5 µl hydrogels, molded between glass slides, are described in the procedure below and recommended to allow rapid diffusion of Ellman's reagent through the gel (i.e., it takes the reagent a longer time to diffuse through thick gels).- Determine the swollen gel volume of 'thin' 5 µl gels (VS) based on the volumetric swelling ratio, Q.
- Make three 20 µl 'thick' gels using syringe molds. Place gels in Ellman's reaction buffer for 24 hr and subsequently weigh (equilibrium swollen mass, MS). Lyophilize the gels and subsequently weigh (dry mass, MD).
- Using the measured masses for thick gels, calculate the volumetric swelling ratio28 by Q = 1 + ρpolymer/ρsolvent(MS/MD-1) where ρpolymer = 1.07 g/cm3 for PEG,29 ρsolvent = 1.0 g/cm3 for PBS/H2O.
- Calculate the theoretical dry mass of the gel to be used for Ellman's Assay (here, 5 µl 'thin' gels are typically used), by summing the masses of individual components PEG4SH, Pep1Alloc, and Pep2Alloc (MD = MPEG4SH + MPep1Alloc + MPep2Alloc). For example, a 5 µl gel containing 10 wt% PEG4SH contains approximately 0.000535 g PEG as calculated by MPEG4SH = 0.005 cm3 x 1.07 g/cm3 x 0.10. Pep1Alloc and Pep2Alloc masses can be calculated in a similar manner based on wt% in solution (see the spreadsheet for values), assuming ρ ≈ 1.0 g/cm3 for these aqueous solutions.
NOTE: Gels also may be dried and weighed instead of calculating the theoretical dry mass. However, it may be challenging to consistently measure the dry mass of the thin, 5 µl gels. - Based on the predicted dry mass and the Q value determined from 'thick' gels, calculate the predicted swollen mass MS for the 'thin' gel (equation in step 3.1.2.1.1). Assume that ρ ≈ 1.0 g/cm3, then MS ≈ VS. Use this calculated VS to perform quantitative Ellman's assay.
NOTE: The above method is recommended to determine Vs for swollen gels as the 'thin' 5 µl gels are difficult to transfer for weighing.
- Form thin hydrogels for patterning as described in section 2.2 (5 µl volume). Specifically, make gels with excess free thiols and 'pattern' half of these samples with Pep1Alloc using a clear coverslip to flood expose the entire gel with light (e.g., 6 total gels with excess thiol: 3 unmodified non-'patterned' and 3 'patterned').
NOTE: For this assay, 'patterned' gels are flood exposed to light without a photomask. This method is used to determine the total number of free thiols consumed in the entire gel sample and to demonstrate how efficiently free thiols may be modified with peptide. From this information, the number of free thiols consumed during patterning with a photomask may be predicted based on the gel thickness and pattern area (regions exposed to light). - Place 'patterned' and non-'patterned' gels in wells of a 48-well plate and rinse 3 x 15 min with Ellman's reaction buffer to allow diffusion of unreacted species out of the gel and equilibrium swelling to occur.
- Add extra Ellman's reaction buffer to the rinsed gels so that Vs with reaction buffer is a multiple of 20 µl. For example, if the predicted VS is 15 µl, add 5 µl reaction buffer (20 µl), and if the predicted VS is 30 µl, add 10 µl reaction buffer (40 µl).
- Pipette the cysteine standards into individual wells (not containing gels) of a 96-well plate in multiples of 20 µl depending on the volume used in the previous step (i.e., if the previous step had VS + extra reaction buffer = 40 µl, add 40 µl of each standard to individual empty wells).
- Dilute Ellman's reagent in reaction buffer (multiples of 180 µl reaction buffer + 3.6 µl Ellman's reagent; e.g., 183.6 for 20 µl or 367.2 µl for 40 µl in step 3.1.2.5).
- Add diluted Ellman's reagent to wells containing standards and samples. For 20 µl samples, add 183.6 µl solution to each well. Double this amount of diluted Ellman's reagent for 40 µl samples (or scale accordingly based on the size of the sample).
- Incubate or place on a shaker for 1 hr and 30 min (at room temperature) or until the color of the solution matches the color of the gels by visual inspection to ensure sufficient diffusion of the yellow 2-nitro-5-thiobenzoate dianion (TNB2-), which is generated upon reaction of Ellman's reagent with free thiols, from the gel.
- Take 100 µl of solution from samples and standards and place into wells of a 96-well plate.
- Read absorbance at 405 nm on a plate reader.
- To process the data, plot the standard curve (samples from step 3.1.1.3) (absorbance versus concentration) and fit a linear function. Using the linear function, the concentration of free thiols in the 'diluted gel' solution (gel + reaction buffer) may be calculated based on absorbance readings. Finally, taking into account the 'dilution' with reaction buffer, determine the free thiol concentration in the gels without reaction buffer. (e.g., if 15 µl gel was diluted with 5 µl reaction buffer, multiply by 20/15).
- Determine the swollen gel volume of 'thin' 5 µl gels (VS) based on the volumetric swelling ratio, Q.
- Visualization of photopatterns with Ellman's reagent (Figure 3A).
- Soak thin hydrogels patterned with Pep1Alloc in Ellman's reaction buffer for 1 hr.
- Remove excess reaction buffer by gently wiping around the edges of the hydrogel with a tissue.
- Pipette Ellman's reagent directly on the surface of the gel. Immediately image on a light microscope at 10X or stereomicroscope with a color camera.
Note: Gels must be imaged immediately because visualization patterns will dissipate within 5 min as the yellow TNB2- ion produced by cleavage of Ellman's reagent upon reaction with thiols in non-patterned region diffuses throughout the gel. Non-patterned regions appear faintly yellow to the naked eye; for better resolution and smaller patterns, magnification (e.g., use of a microscope) is required.
- Prepare Ellman's reaction buffer, cysteine working solution, standards, and Ellman's reagent as described below.
- Visualization of Photopatterns with Fluorescent Peptides (Figure 3B).
- For visualizing patterns with higher resolution or in three-dimensions, photopattern an AF488-modified Pep1Alloc (or similar fluorescent peptide) to the gels in step 2.2.
- Image on a fluorescent microscope. A confocal microscope may be used here to take z-stack images of the gel so that the pattern may be observed in the x-, y-, and z-planes.
NOTE: With fluorescence, gels must be protected from light to ensure the stability of the AF488 fluorophore and maximum fluorescence. Gels do not need to be immediately imaged since the fluorescent peptide is fairly stable if protected from light (store in a container wrapped in foil at 4 °C).
4. Cell Encapsulation in Hydrogels and Photopatterning
- Collecting and Preparing Cells for Encapsulation.
- Following standard sterile mammalian cell culture procedures, trypsinize and collect cells of interest from plates. Quench the trypsin with growth medium after detachment occurs (e.g., for a T-75 flask, 5 ml trypsin, quench with 5 ml medium, rinse plate with 5 ml medium for total 15 ml).
NOTE: Trypsinization times can vary between cell types but typically occur between 5 and 15 min. Alternate cell detachment agents (e.g., Versene) may be used to collect cells if desired. - Count cells (a minimum of 100 or per manufacturer's protocol) from aliquots of the trypsinized cell suspension using a hemocytometer or other cell counting device while centrifuging the bulk cell suspension (90-110 x g, 5 min). Re-suspend cell pellet after centrifugation in a minimal volume of PBS or growth medium and re-count if the initial trypsinized cell suspension is too dilute.
- Re-suspend cells in a minimal volume of PBS and aliquot portions for each gel condition into microcentrifuge tubes so that there will be 5,000 cells/µl when mixed with the gel solution (prior to polymerization).
NOTE: Typical cell aliquots contain 300,000-500,000 cells and are enough to make 60-100 µl of gel/cell suspension which can be used for 3-5 x 20 µl gels with 5,000 cells/µl. Higher or lower cell densities may be used for encapsulation, depending on the amount of cell-cell vs. cell-matrix contact desired, respectively, and should be determined for each application. - Centrifuge cell/PBS aliquots (90-110 x g, 5 min).
- Carefully aspirate PBS from cell pellet in microcentrifuge tube right before encapsulation.
NOTE: If cells are sensitive to shear forces, the second centrifugation step may be eliminated by counting (e.g., hemocytometer) and subsequently aliquotting the trypsinized cell suspension (trypsin + media + cells) into portions needed for each gel condition. These aliquots are centrifuged once (90-110 x g, 5 min) and the trypsin + media is aspirated off, leaving a cell pellet for encapsulation.
- Following standard sterile mammalian cell culture procedures, trypsinize and collect cells of interest from plates. Quench the trypsin with growth medium after detachment occurs (e.g., for a T-75 flask, 5 ml trypsin, quench with 5 ml medium, rinse plate with 5 ml medium for total 15 ml).
- Encapsulating Cells in Non-patterned Hydrogels.
- Immediately after aspirating PBS, suspend pelleted cells in PEG4SH, Pep2Alloc, Pep1Alloc, LAP, and PBS+PS+FZ as calculated in the spreadsheet to a final concentration of 5,000 cells/µl.
- Mold and polymerize hydrogels as described in steps 2.1.2 to 2.1.6.
NOTE: When encapsulating cells in glass slide molds, care must be taken when removing the top slide post-polymerization to prevent shearing the gel, which can result in cell death. To help with removal of the gel from the mold, slide molds may be placed in sterile PBS or growth medium after polymerization to wet the gasket and hydrogel, making it easier to remove the top slide. A method to prevent this shear altogether is through the use of syringe molds. - Rinse gels 3 x 10 min in growth medium to remove unreacted species and excess LAP.
- Incubate gels in growth medium at 37 °C and 5% CO2 until the desired time point for further analysis. Replenish medium every 48 hr or as determined for the application (e.g., typical feeding interval for specific cell type and medium).
- Encapsulating Cells and Photopatterning in the Presence of Cells.
- Immediately after aspirating PBS, suspend pelleted cells in PEG4SH, Pep2Alloc, LAP, and PBS+PS+FZ as calculated in the spreadsheet to a final concentration of 5,000 cells/µl. Leave an appropriate concentration of free thiols (e.g., 2 mM) for later reaction with Pep1Alloc.
- Mold, polymerize, and pattern hydrogels as described in steps 2.2.2 to 2.2.8.
- Rinse gels 3 x 10 min in growth medium after patterning to remove unreacted species and excess LAP.
- Incubate gels in growth medium at 37 °C and 5% CO2 until the desired time point for further analysis. Replenish medium every 48 hr or as determined for the application.
5. Determining the Viability and Metabolic Activity of Encapsulated Cells
- Perform Live/Dead Cytotoxicity Assay to Determine Encapsulated Cell Viability (Figure 4A).
- Remove growth medium from gels and rinse 3 x 15 min with PBS to allow diffusion of medium from gels.
- Thaw live/dead cytotoxicity assay solutions (ethidium homodimer-1 and calcein AM).
- Add 2 µl of the 2 mM ethidium homodimer-1 and 0.5 µl of the 4 mM calcein AM solutions to 1 ml sterile PBS. Vortex to ensure complete mixing.
- Add 300 µl staining solution to each syringe mold gel in 48-well plates, or enough to cover the surface of gel on a glass slide in a sterile dish, and incubate for 45 min.
- Remove excess staining solution and rinse to remove excess dye from the gels (3 x 15 min with PBS).
- Image on a confocal microscope (z-stack images) or on an epi-fluorescence microscope with z-stack capability at 10X and 488/525 nm Ex/Em. Quantify the number of viable cells by projecting the z-stacks and counting the number of live (green throughout cell body) and dead (red nuclei) cells with imaging software.
- Perform metabolic activity assay to determine cell activity post-encapsulation (Figure 4B).
- 24 hr prior to assay, feed encapsulated cells with fresh growth medium.
NOTE: Add medium to a few extra wells (containing no gels or gels with no cells) as control (background readings). - At the time point of interest, thaw MTS reagent solution.
NOTE: In order to determine metabolic activity over time, an initial time point (12-24 hr post-encapsulation) is recommended as a reference for comparison to later time points. - Add MTS reagent to each well (20 µl per 100 µl growth medium).
- Incubate samples for 1-4 hr at 37 °C and 5% CO2, per manufacturer's instructions.
NOTE: Incubation times should be sufficient for cells to reduce MTS and allow diffusion of the formazan product out of the gel. Consequently, thicker gels such as syringe tip gels may require longer incubation times (~4 hr) for sufficient MTS reduction and diffusion. - Pipette 100 µl reduced MTS/Medium into clean wells of a 96-well plate and record absorbance at 490 nm on a plate reader.
- Subtract the background absorbance for wells without cells from sample absorbance values to generate baselined absorbance values.
- 24 hr prior to assay, feed encapsulated cells with fresh growth medium.
Representative Results
The setup and procedure to photoencapsulate cells and subsequently photopattern gels containing encapsulated cells is depicted in Figure 1, and an example for preparing stock solutions to form a 10 wt% gel is provided in Table 1. Using Table 1, the amount of monomers (PEG4SH, Pep2Alloc, ±Pep1Alloc) and photoinitiator (LAP) required to polymerize hydrogels is calculated. Based on these calculations, stock solutions of PEG4SH, Pep1Alloc, Pep2Alloc, and LAP are prepared and mixed with and without Pep1Alloc for forming and photopatterning gels, respectively (Figure 1A). Subsequently, cells are collected from plates, counted, and centrifuged in appropriate quantities for encapsulation (Figure 1B). The cell pellet is re-suspended in gel-forming solution (peptide/polymer/LAP in PBS), and cell/monomer mixtures are transferred to glass or syringe molds. Cells are encapsulated within the hydrogel upon application of light (1-5 minutes of 10 mW/cm2 at 365 nm) (Figure 1C). For photopatterning (Figure 1D), gels are soaked with Pep1Alloc and LAP for 30 min to 1 hr and 30 min, allowing diffusion of peptide and initiator into the polymerized matrix. These peptide-laden gels are covered with photomasks with desired patterns and exposed to a second dose of light (1 min) to conjugate the peptides to free thiols within the matrix. Pendant tethers are only covalently linked to the gel in regions exposed to light, facilitating appropriate cell-matrix interactions and mimicking key mechanical and biochemical properties of the native cell microenvironment toward probing cell function and fate in vitro.
Ellman's assay provides a facile and inexpensive method to quantify gel modification and peptide incorporation within photopatterned substrates. Patterned and non-patterned gels (i.e., gels with or without peptide modification) are soaked in Ellman's reaction buffer post-polymerization. Next, cysteine standards and the gels soaked in buffer are placed in 48-well plates and incubated with Ellman's reagent (Figure 2A, left). After 1 hour 30 minutes, aliquots of samples are placed in individual wells of a 96-well plate, and the absorbance is recorded at 405 nm. A calibration curve (Figure 2A, right) from the cysteine standards is plotted (absorbance vs concentration, linear fit), and the quantity of free thiols in gels may be determined based on their dilution factor. These free thiol concentrations for various polymerization and photopatterning conditions of a 10 wt% gel are shown in Figure 2B. Gels polymerized for 1 or 5 min without Pep1Alloc (blue bars, I = 1 min and II = 5 min) have statistically similar free thiol concentrations, indicating that a rapid reaction occurs and gelation is complete within 1 min (two-tailed t-test, p >0.05). Thus, additional exposure to light (2-5 minutes) does not result in further conversion of functional groups. Gels polymerized for 1 min without Pep1Alloc were soaked with Pep1Alloc (3 mg/ml) and LAP (2.2 mM) for 30 and 90 min (green bars, II = 30 min and IV = 90 min) and exposed to a second dose of light for 1 min. The decrease in free thiols (60-80% with respect to the 1 min condition) indicates efficient modification of gels with pendant cues under these conditions. If higher modification is desired, increased concentrations of Pep1Alloc solution can be used as accessibility of Pep1Alloc to free thiols in more dilute peptide solutions may limit conversion; for example, we have found that concentrations up to 20 mg/ml Pep1Alloc produce >90% conversion of free thiols.
Uniquely, patterns of peptides added to the hydrogel may be rapidly imaged with Ellman's reagent (Figure 3A, under 5 min). However, visualization of the pattern is lost over time (greater than 5 min) owing to diffusion of the yellow TNB2- dianion through the gel. To improve imaging resolution and observe patterns in three dimensions (x-, y-, and z-planes) and in the presence of cells, fluorescent peptide addition (AF488Pep1Alloc) may be utilized. In Figure 3B x-, y- and z-projections of image stacks taken with a confocal microscope are shown, demonstrating pattern resolution (µm scale).
Approximately (94 ± 2) % and (94 ± 1) % of hMSCs encapsulated within degradable gels (Pep2Alloc = GPQG↓WGQ) remain viable (green cell bodies) 1 and 6 days after encapsulation, respectively, with few dead cells (red nuclei) observed (Figure 4A). Further, hMSC spreading is observed at 6 days post-encapsulation (Figure 4A, inset), indicating that cells can remodel and interact with these MMP-degradable matrices modified with integrin-binding RGDS. Metabolic activity assays performed on cells encapsulated in non-degradable gels (Pep2Alloc = RGKGRK) 1 and 3 days post-encapsulation (Figure 4B) provide a second measure of cell viability and demonstrate that cells remain active for the various photoencapsulation and photopatterning conditions tested with Ellman's assay (Figure 2B). In particular, there is no significant difference in metabolic activity between 30 min and 90 min incubations with Pep1Alloc+LAP (conditions III and IV) and the initial encapsulation (conditions I and II), indicating that the procedure is appropriate for applications of photopatterning in the presence of encapsulated cells (two-tailed t-test, p > 0.05).
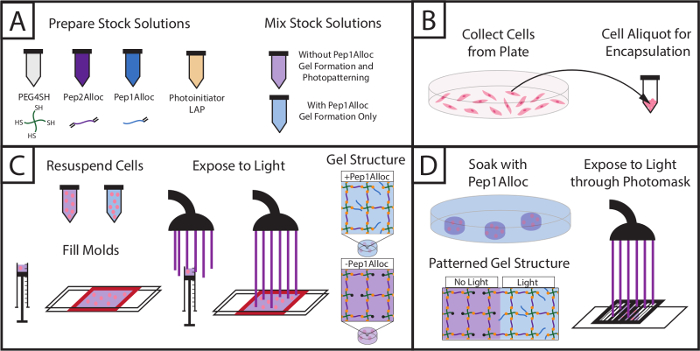
Figure 1: Setup for encapsulating cells within hydrogels and subsequently photopatterning with biochemical cue. (A) Stock solutions of macromers and photoinitiator are prepared and mixed (PEG4SH = backbone, Pep2Alloc = crosslink, Pep1Alloc = pendant adhesive moiety (RGDS), LAP = photoinitiator). (B) Cells are collected for encapsulation. (C) Cells are mixed with macromer solutions without or with integrin-binding peptides (PEG4SH/Pep2Alloc/LAP or PEG4SH/Pep2Alloc/Pep1Alloc/LAP, respectively) and are encapsulated upon exposure to light. (D) Gels containing excess thiol groups during gel formation (here, PEG4SH/Pep2Alloc/LAP, formed with 2 mM excess thiol) may be patterned with biochemical cues by the subsequent addition of peptides functionalized with a single alloc group (Pep1Alloc, e.g., RGDS, IKVAV, etc.) to promote cell adhesion within specific regions of the gel. Here, patterning of gels with fluorescently-labeled RGDS is shown. Please click here to view a larger version of this figure.
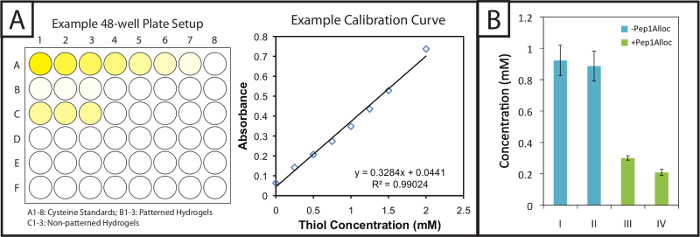
Figure 2: Quantitative Ellman's assay setup and results to evaluate modification of patterned hydrogels. (A) Gels and cysteine standards are incubated in 48-well plates with Ellman's reagent. A linear standard curve is plotted to determine cysteine concentration in gel samples. (B) Excess free thiols are incorporated within hydrogels during gel formation and consumed upon patterning with a pendant alloc-peptide (I = 1 min polymerization; II = 5 min polymerization; III = 30 min incubation with Pep1Alloc; IV = 90 min incubation with Pep1Alloc; both III and IV polymerized and patterned with light for 1 min). The data shown illustrate the mean (n = 3) with error bars showing the standard error. Please click here to view a larger version of this figure.
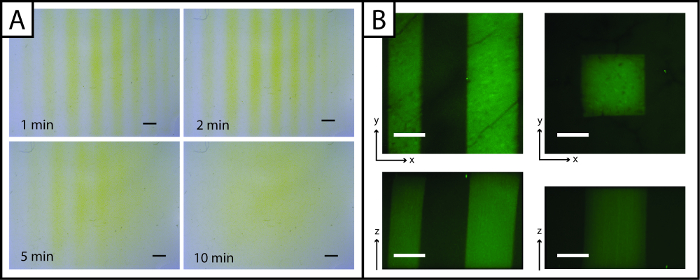
Figure 3: Ellman's Assay and fluorescent images to visualize photopatterned hydrogels. (A) Ellman's reagent may be used to rapidly detect patterns (lines) in the x- and y-planes (yellow = unpatterned region, Scale bar = 1 mm). (B) Fluorescent peptides may be used to observe patterns in the x-, y-, and z-planes (green = patterned region; 200 µm scale bar; 10X water-dipping objective; Ex/Em 488/525 nm). Please click here to view a larger version of this figure.
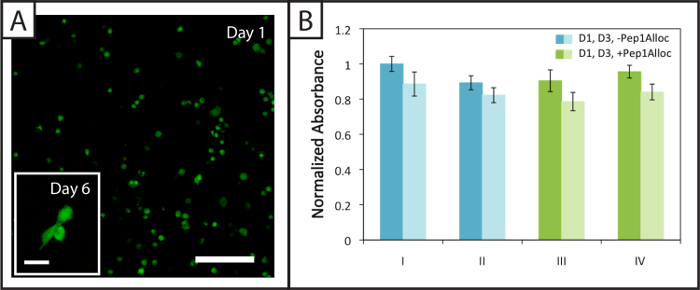
Figure 4: Viability and metabolic activity of cells within non-degradable photopatterned hydrogels. (A) Example confocal z-stack (z-projection; 10X water-dipping objective) of viable (green; Ex/Em 488/525 nm) and dead hMSCs (red; Ex/Em 543/580 nm) encapsulated within hydrogels 24 hr post-encapsulation (Scale bar = 200 µm). Cells spread within these hydrogels 6 days after encapsulation (inset image, Scale bar = 50 µm). (B) Cells are metabolically active 1 and 3 days (dark and light bars, respectively) post-encapsulation for the various polymerization (I = 1 min polymerization; II = 5 min polymerization) and patterning conditions (III = 30 min incubation with Pep1Alloc; IV = 90 min incubation with Pep1Alloc; both polymerized and patterned with light for 1 min). The data shown illustrate the mean (n = 3) with error bars showing the standard error. Please click here to view a larger version of this figure.

Table 1: Example setup to calculate volumes of stock solutions for making hydrogels. Cells within the spreadsheet that are highlighted blue indicate user-defined parameters; the other quantities are calculated based on these settings. The final volumes for each stock solution to make a gel are highlighted orange. The white cells contain formulas used to calculate the final volumes based on the user-defined parameters. Note that a concentration of 2.2 mM LAP is equivalent to 0.067 wt%.
Discussion
The procedure presented here demonstrates techniques to photoencapsulate cells within hydrogels formed by thiol−ene click chemistry and subsequently photopattern the gels with biochemical cues. The use of light to initially form hydrogels allows homogeneous mixing and suspension of the cells within the polymer solution prior to polymerization. Rapid polymerization 'locks' the gel in the shape of the defined mold and encapsulates suspended cells within the hydrogel network. Gels also may be molded into numerous different shapes (e.g., glass slides or syringe tips) depending on the final application desired. For example, cells encapsulated for 3D culture in hydrogels attached to glass slides are particularly useful for imaging applications as light attenuation is limited within a thin sample. Syringe molds can be used for rapid encapsulation of cells, allowing a larger number of samples to be prepared in a short time (compared to glass slides) that can be used for experiments requiring large numbers of cells such as flow cytometry or qPCR. Subsequently, these gels then may be patterned with biochemical cues to elicit desired cellular responses such as differentiation or invasion.30,31
Assays for viability and metabolic activity indicate the survival of cells for the material system and patterning conditions presented. Note that metabolic activity was monitored until day 3 in non-degradable gels (RGKGRK peptide crosslink) to assess the initial effects of polymerization and patterning conditions on cell function. Additionally, a membrane integrity assay (live/dead viability/cytotoxicity staining) of hMSCs at 1 and 6 days post-encapsulation in gels formulated with a degradable peptide crosslink (GPQGIWGQ) supports that cells remain viable and spread at 1 week in culture. The viability of additional cell lines has been reported for photopatterning conditions32 similar to those used here and can be evaluated for the described hydrogel system using live/dead staining and metabolic activity assays. While we have not observed issues with cell viability using this materials system and related procedures (4.2 and 4.3), some cell types may be sensitive to free-radical and/or light exposure. In this case, users may consider using non-photoinitiated materials systems, such as azide-alkyne,12 FXIII,31 or Diels Alder-based hydrogel formation chemistries.33
Facile techniques to detect and quantify patterning of biochemical cues within gels also are presented (3.1 and 3.2). Ellman's assay is of particular interest because the reagents are commercially available and no extra synthetic processing steps or more expensive reagents (e.g., fluorescently-labeled peptide) are required. Ellman's assay can be used to precisely determine the modification of free thiols with biochemical cues under different photopatterning conditions, as well as to rapidly visualize patterns. For quantifying peptide incorporation, the thiol functional group concentration before and after patterning, as a quantitative measure of peptide incorporation, is directly assessed with Ellman's assay. While this type of quantification can be done with fluorescently-labeled peptides,34 imaging-based quantification requires more time-consuming handling and analysis steps (e.g., synthesis of a fluorescently-labeled peptide and generation of a calibration curve to relate fluorescence to peptide concentration using image analysis). For imaging peptide incorporation, Ellman's reagent can be directly applied to samples and immediately visualized. While limited to the x- and y-planes for pattern visualization, the technique can be used as a simple, routine method to determine if matrices containing free thiol groups have been patterned. It is important to note that Ellman's assay is not considered cytocompatible, so while it may be used to observe and quantify photopatterns, it cannot be done in the presence of cells. For imaging patterning in three dimensions and in the presence of cells, the conjugation of fluorescent peptides within hydrogel matrices remains a powerful and widely-used approach. Resolution of patterns can be evaluated in the x-, y-, and z-planes using confocal microscopy, and this method is cytocompatible so that cells within patterned regions or non-patterned regions can be identified. Taken together, Ellman's assay and imaging-based techniques are complementary tools for researchers to assess both quantitatively and qualitatively the photopatterning of biochemical cues within the materials system.
Photoclick, or more broadly photoinitiated, chemistries for the formation and modification of hydrogels in the presence of cells are numerous. The molding, encapsulation, and patterning techniques presented here are not limited to the described material system and may be applied to alternative light-based chemistries, such as thiol-alkyne,35 azide-alkyne,4 and other thiol−ene chemistries (e.g., thiol-norbornene),10,13 as well as with different photoinitiators, such as Irgacure 2959, Eosin Y, and camphorquinone. Note, users may need to adjust procedure parameters (e.g., incubation times, polymerization times, cell density) to ensure that conditions remain cytocompatible for these other systems. Since the patterning process requires diffusion of the alloc-modified peptide(s) into the hydrogel (2.2.3-2.2.5), this process may prove most useful for the addition of integrin-binding moieties (e.g., peptides or extracellular matrix protein fragments) to the hydrogel, where attachment of the ligand to the network is required for the generation of traction forces by the cell and full integrin activation.36 Note, for biomolecules that may be similarly active in solution or upon immobilization (e.g., growth factors or cytokines), the incubation step for moiety diffusion (~1 hr) into the hydrogel could lead to signaling events that convolute patterning results. Other methods have been established for growth factor immobilization or local sequestration for their patterning.37-39Additionally, pattern resolution is dictated by the control over light exposure. Here, photomasks allow creation of patterns through the gel depth and in the x- and y-planes; however, greater spatial control over patterning of biochemical cues within gels can be achieved with alternative methods of irradiation such as the use of a two-photon confocal microscope to generate patterns in the x-, y-, and z- planes.34,40 Finally, it is important to note that while the material system utilized within this procedure is only initially modified with biochemical cues, orthogonal photoclick chemistries could be used to allow alterations in matrix properties over time.12 The procedure and techniques presented here add diversity to the current approaches for creating synthetic matrices with well-defined and spatiotemporally-controlled properties. In particular, the commercial availability of reagents and materials used within this procedure will be useful to a wide range of researchers interested in the use of hydrogel-based biomaterials for applications in controlled cell culture.
Divulgaciones
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Delaware COBRE programs in Drug Discovery and in Advanced Biomaterials funded by Institutional Development Awards from the National Institute of Generals Medical Sciences at the National Institutes of Health (P20GM104316 and P30 GM110758-01, respectively), the Pew Charitable Trusts (00026178), a National Science Foundation Career Award (DMR-1253906), the Burroughs Wellcome Fund (1006787), and the National Science Foundation IGERT SBE2 program at the University of Delaware (fellowship to L. Sawicki). The authors thank the Delaware Biotechnology Institute BioImaging Center at the University of Delaware for training and access to confocal microscopy, Ms. Katherine Wiley for assistance during the video shoot, Mr. Matthew Rehmann for generously providing hMSCs isolated from bone marrow, Prof. Christopher J. Kloxin and Mr. Stephen Ma for generously providing photomasks, and Prof. Wilfred Chen for the use of the automated plate reader.
Materials
| Custom Peptides | Various Vendors | —- | Peptides may also be synthesized via standard SPPS techniques with materials from vendors including ChemImpex and ChemPep. |
| 4arm PEG Thiol, MW 20k | JenKem USA | 4ARM-SH | Listed under Multi-arm Homofunctional PEGs. PEG4SH may also be synthesized as previously referenced. |
| Lithium Phenyl-2,4,6-trimethylbenzoylphosphinate | Colorado Photopolymer Solutions | Li-TPO | |
| Dimethyl Phenylphosphonite | Sigma Aldrich | 149470 | Caution, causes severe skin burns and eye damage. Wear protective gloves, clothing, and eye protection. |
| 2.4.6-Trimethylbenzoyl Chloride | Sigma Aldrich | 682519 | Caution, causes severe skin burns and eye damage. Wear protective gloves, clothing, and eye protection. |
| Lithium Bromide | Sigma Aldrich | 213225 | |
| 2-Butanone | Sigma Aldrich | 360473 | |
| Flask, Round Bottom, 100 mL | Chemglass | CG-1506-05 | |
| 80 mL Filter Funnel, Buchner, Medium Frit | Chemglass | CG-1402-L-02 | Filter paper inside a regular glass funnel may be used if desired. |
| Magnetic Stir Bars | Various Vendors | —- | |
| Magnetic Stirring and Hot Plate | Various Vendors | —- | |
| Vacuum Dessicator | Various Vendors | —- | |
| Deuterium Oxide | Acros Organics | 16630 | |
| Dulbecco's Phosphate Buffered Saline | ThermoFisher Scientific | 14190-250 | |
| Penicillin Streptomycin | ThermoFisher Scientific | 15070-063 | |
| Fungizone | ThermoFisher Scientific | 15290-018 | |
| Trypsin-EDTA (0.5%), no phenol red | ThermoFisher Scientific | 15400-054 | Select appropriate medium and trypsin depending on cell type. |
| Microcentrifuge tubes | Various Vendors | —- | 1.5 or 2 mL sizes, sterile. |
| BD Syringe with Slip (Luer) Tips (Without Needle) | Fisher Scientific | 14-823-16H | Product number listed here is for a 1 mL syringe (16H), Various sizes are available (14-823-XX). |
| Fisherfinest Premium Plain Glass Microscope Slides | Fisher Scientific | 12-544-1 | |
| High-Purity Silicone Rubber, 0.010" Thick, 6" x 8" Sheet, 55A Durometer (Gasket) | McMaster Carr | 87315K62 | |
| Ethanol, 200 Proof | Decon Labs | 2701 | |
| RainX, Original | Amazon | 800002243 | May be purchased from other vendors. |
| Sigmacote | Sigma Aldrich | SL2 | |
| Photomasks | Advance Reproductions Corporation | —- | Photomask Division, different designs may be printed as desired. |
| DTNB; Ellman's Reagent; 5,5-dithio-bis(2-nitrobenzoic acid) | ThermoFisher Scientific | PI-22582 | |
| Sodium Phosphate Dibasic | Sigma Aldrich | S5136 | |
| Ethylenediaminetetraacetic acid | Sigma Aldrich | E6758 | |
| Sodium Hydroxide, Pellets/Certified ACS | Fisher Scientific | S318 | |
| Orthophosphoric Acid | Alfa Aesar | 33266 | |
| Cysteine Hydrochloride Monohydrate | Sigma Aldrich | C7880 | |
| LIVE/DEAD Viability/Cytotoxicity Kit, for mammalian cells | ThermoFisher Scientific | L-3224 | |
| CellTiter 96 Aqueous One Solution Assay | Promega | G3582 | |
| Centrifuge | Various Vendors | —- | Capable of speeds at 90-110 x g. |
| Multiwell Plate Reader | Various Vendors | —- | Capable of reading absorbance at 405 nm in a 96-well plate. |
| Epifluorescent or Confocal Microscope | Various Vendors | —- | To visualize peptide patterns and cells within hydrogels. |
| Omnicure Exfo Series 2000 | Excelitas Technologies | —- | Alternate light systems may be used to polymerize hydrogels. |
| Zeiss Zen Lite Software | Zeiss | —- | Available at zeiss.com; compatible with images taken on Zeiss microscopes |
| ImageJ | NIH | —- | Available at imagej.nih.gov; applicable for general image analysis |
Referencias
- Kolb, H. C., Finn, M. G., Sharpless, K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chemie – Int Ed. 40 (11), 2004-2021 (2001).
- Xi, W., Scott, T. F., Kloxin, C. J., Bowman, C. N. Click Chemistry in Materials Science. Adv Funct Mater. 24 (18), 2572-2590 (2014).
- Azagarsamy, M. A., Anseth, K. S. Bioorthogonal click chemistry: An indispensable tool to create multifaceted cell culture scaffolds. ACS Macro Lett. 2 (1), 5-9 (2013).
- Adzima, B. J., Tao, Y., Kloxin, C. J., DeForest, C. A., Anseth, K. S., Bowman, C. N. Spatial and temporal control of the alkyne-azide cycloaddition by photoinitiated Cu(II) reduction. Nat Chem. 3 (3), 256-259 (2011).
- Hoyle, C. E., Bowman, C. N. Thiol-ene click chemistry. Angew Chemie – Int Ed. 49 (9), 1540-1573 (2010).
- Fan, Y., Deng, C., Cheng, R., Meng, F., Zhong, Z. In situ forming hydrogels via catalyst-free and bioorthogonal "tetrazole-Alkene" photo-click chemistry. Biomacromolecules. 14 (8), 2814-2821 (2013).
- Burdick, J. A., Murphy, W. L. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 3, 1269 (2012).
- Rehmann, M. S., Kloxin, A. M. Tunable and dynamic soft materials for three-dimensional cell culture. Soft Matter. 9 (29), 6737-6746 (2013).
- Yang, T., Long, H., Malkoch, M., Gamstedt, K. E., Berglund, L., Hult, A. Characterization of well-defined poly(ethylene glycol) hydrogels prepared by thiol-ene chemistry. J Polym Sci Part A Polym Chem. 49 (18), 4044-4054 (2011).
- Fairbanks, B. D., et al. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater. 21 (48), 5005-5010 (2009).
- Roberts, J. J., Bryant, S. J. Comparison of photopolymerizable thiol-ene PEG and acrylate-based PEG hydrogels for cartilage development. Biomaterials. 34 (38), 9969-9979 (2013).
- DeForest, C. A., Polizzotti, B. D., Anseth, K. S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 8 (8), 659-664 (2009).
- Lin, C. C., Ki, C. S., Shih, H. Thiol-norbornene photoclick hydrogels for tissue engineering applications. J Appl Polym Sci. 132 (8), (2015).
- Sawicki, L. A., Kloxin, A. M. Design of thiol-ene photoclick hydrogels using facile techniques for cell culture applications. Biomater Sci. 2 (11), 1612-1626 (2014).
- Patterson, J., Hubbell, J. A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 31 (30), 7836-7845 (2010).
- Fairbanks, B. D., Singh, S. P., Bowman, C. N., Anseth, K. S. Photodegradable, photoadaptable hydrogels via radical-mediated disulfide fragmentation reaction. Macromolecules. 44 (8), 2444-2450 (2011).
- Fairbanks, B. D., Schwartz, M. P., Bowman, C. N., Anseth, K. S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 30 (35), 6702-6707 (2009).
- Yang, F., et al. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 26 (30), 5991-5998 (2005).
- Wacker, B. K., et al. Endothelial cell migration on RGD-peptide-containing PEG hydrogels in the presence of sphingosine 1-phosphate. Biophys J. 94 (1), 273-285 (2008).
- Wilson, M. J., Liliensiek, S. J., Murphy, C. J., Murphy, W. L., Nealey, P. F. Hydrogels with well-defined peptide-hydrogel spacing and concentration: impact on epithelial cell behavior. Soft Matter. 8 (2), 390-398 (2012).
- Qiong Liu, S., et al. Injectable biodegradable polyethylene glycol/ RGD peptide hybrid hydrogels for in vitro chondrogenesis of human mesenchymal stern cellsa. Macromol Rapid Commun. 31 (13), 1148-1154 (2010).
- Engler, A. J., Sen, S., Sweeney, H. L., Discher, D. E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 126 (4), 677-689 (2006).
- Levental, I., Georges, P. C., Janmey, P. A. Soft biological materials and their impact on cell function. Soft Matter. 3 (3), 299-306 (2007).
- Lin, C. C., Raza, A., Shih, H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials. 32 (36), 9685-9695 (2011).
- Khetan, S., Burdick, J. Cellular encapsulation in 3D hydrogels for tissue engineering. J Vis Exp. (32), e1590 (2009).
- Bryant, S. J., Nuttelman, C. R., Anseth, K. S. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 11 (5), 439-457 (2000).
- Burdick, J. A., Chung, C., Jia, X., Randolph, M. A., Langer, R. Controlled Degradation and Mechanical Behavior of Photopolymerized Hyaluronic Acid Networks Controlled Degradation and Mechanical Behavior of Photopolymerized Hyaluronic Acid Networks. Society. 6 (1), 386-391 (2005).
- Marsano, E., Gagliardi, S., Ghioni, F., Bianchi, E. Behaviour of gels based on (hydroxypropyl) cellulose methacrylate. Polymer (Guildf). 41 (21), 7691-7698 (2000).
- Bryant, S. J., Anseth, K. S. Photopolymerization of Hydrogel Scaffolds. Scaffolding Tissue Eng. , 71-90 (2005).
- Khetan, S., Burdick, J. A. Patterning hydrogels in three dimensions towards controlling cellular interactions. Soft Matter. 7 (3), 830-838 (2011).
- Mosiewicz, K. A., et al. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat Mater. 12 (11), 1072-1078 (2013).
- Williams, C. G., Malik, A. N., Kim, T. K., Manson, P. N., Elisseeff, J. H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 26 (11), 1211-1218 (2005).
- Nimmo, C. M., Owen, S. C., Shoichet, M. S. . Diels – Alder Click Cross-Linked Hyaluronic Acid Hydrogels for Tissue Engineering. , 824-830 (2011).
- DeForest, C. A., Anseth, K. S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 3 (12), 925-931 (2011).
- Fairbanks, B. D., Sims, E. A., Anseth, K. S., Bowman, C. N. Reaction rates and mechanisms for radical, photoinitated addition of thiols to alkynes, and implications for thiol-yne photopolymerizations and click reactions. Macromolecules. 43 (9), 4113-4119 (2010).
- Tibbitt, M. W., Anseth, K. S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 103 (4), 655-663 (2009).
- Wylie, R. G., et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat Mater. 10 (10), 799-806 (2011).
- Pompe, T., Salchert, K., Alberti, K., Zandstra, P., Werner, C. Immobilization of growth factors on solid supports for the modulation of stem cell fate. Nat Protoc. 5 (6), 1042-1050 (2010).
- Hudalla, G. A., Murphy, W. L. Biomaterials that regulate growth factor activity via bioinspired interactions. Adv Funct Mater. 21 (10), 1754-1768 (2011).
- Lee, S. H., Moon, J. J., West, J. L. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials. 29 (20), 2962-2968 (2008).

